PVP-crosslinked electrospun membranes with embedded Pd and Cu2O nanoparticles as effective heterogeneous catalytic supports
Ioanna Savvaa,
Andreas S. Kalogiroub,
Andrea Chatzinicolaoua,
Petri Papaphilippoua,
Athena Pantelidoua,
Eugeniu Vasilec,
Eugenia Vasiled,
Panayiotis A. Koutentisb and
Theodora Krasia-Christoforou*a
aDepartment of Mechanical and Manufacturing Engineering, University of Cyprus, P.O. Box 20537, 1678 Nicosia, Cyprus. E-mail: krasia@ucy.ac.cy
bDepartment of Chemistry, University of Cyprus, P.O. Box 20537, 1678 Nicosia, Cyprus
cUniversity Politehnica of Bucharest, Faculty of Applied Chemistry and Material Science, Department of Oxide Materials and Nanomaterials, No. 1-7 Gh. Polizu Street, 011061 Bucharest, Romania
dUniversity Politehnica of Bucharest, Faculty of Medical Engineering, Department of Bioengineering and Biotechnology, No 133 Splaiul Independentei Street, 060042, Bucharest, Romania
First published on 8th September 2014
Abstract
Palladium(0) (Pd) and copper(I) oxide (Cu2O) nanoparticles (NPs) were successfully embedded in electrospun polyvinylpyrrolidone (PVP) fibrous membranes. The fabrication process involved the synthesis of stable, PVP-capped Pd and Cu2O colloidal hybrid solutions in methanol that on subsequent electrospinning afforded PVP–Pd and PVP–Cu2O fibrous mats. The morphology of the as-prepared nanocomposite fibers was characterised using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). SEM revealed the presence of bead-free, cylindrical fibers with diameters in the submicrometer range while TEM revealed the presence of spherical Pd and Cu2O NPs with diameters below 10 nm that were evenly distributed within the fibers. Thermal treatment of the PVP–Pd and the PVP–Cu2O membranes afforded crosslinked fibrous mats as supported by SEM. Furthermore, the presence of homogeneously distributed Pd and Cu2O NPs within the crosslinked polymer fibers was confirmed by HRTEM/EDX analyses. The above-mentioned nanocomposite fibers demonstrated high catalytic efficacy as heterogeneous catalytic supports in Heck, Suzuki (PVP–Pd) and click (PVP–Cu2O) reactions. Finally, the reusability of the membranes was briefly investigated with up to three consecutive runs being effective.
1. Introduction
Electrospinning is a simple, cost-effective technique used for the production of fibrous materials comprised of long, continuous fibers with diameters ranging from a few nanometers up to a few micrometers.1–3 While this process dates back to 1900,4 the first scientific publications on electrospun nanofibers appeared only in the early 1990s with the term “electrospinning” being popularised by Reneker.5–7 The process is versatile and enables not only the fabrication of pristine polymer fibers but also of fibrous nanocomposites via the combination of polymers with inorganic nanofillers. This versatility has led to an exponential increase in the number of related publications, reaching to date around 2000 per year.8 These numbers reflect the importance of the electrospinning process, which, when combined with the current state of art can enhance the development of advanced, polymer-based fibrous nanocomposites destined for use in various technological applications. Among such nanoadditives, metal and metal oxide nanoparticles (NPs) with their unique crystalline structures and large specific surface areas often exhibit excellent catalytic properties.9–14 It is anticipated that the combination of the catalytic properties of NPs, with the unique characteristics of electrospun fibrous materials (e.g., high surface-to-volume ratio, high porosity, ease of chemical/physical post-modification processes) will lead to the development of new, multifunctional fibrous nanocomposites with outstanding catalytic behaviour. Moreover, the immobilization of inorganic catalytic centers within an insoluble polymer matrix prevents nanoparticle aggregation that can reduce catalytic efficiency, and enables the catalyst's facile recovery and reuse. In contrast, analogous NP-containing colloidal systems cannot easily be separated from reaction products, which limits their use in practical applications.15Electrospun fibers comprised of solely inorganic materials16–21 or carbon nanofiber/nanotube-based electrospun systems22–24 have been fabricated and evaluated in catalytic processes. However, there are only a few reports on the fabrication of polymer-NP electrospun fibrous nanocomposites and their evaluation in heterogeneous catalysis: Pd(0) metal nanoparticles (MNPs) were incorporated in electrospun polymer fibrous matrices and evaluated as catalytic supports in Heck24 and hydrogenation15,26,27 reactions; and Au or Ag MNPs were incorporated into fibrous electrospun mats and the resulting fibrous nanocomposites evaluated in reduction,28,29 and electrocatalytic oxidation processes.30
Herein, we describe the fabrication of PVP–Pd and PVP–Cu2O electrospun fibrous nanocomposite membranes and their evaluation as heterogeneous catalytic supports in Heck, Suzuki and click chemistry reactions.
While two recent reports describe the evaluation of PVP–Pd electrospun fibers in Heck reaction processes,15,25 no other coupling reactions have appeared in the literature using this type of polymer-based catalytic support. In the present work the PVP–Pd electrospun fibrous nanocomposite membranes were investigated in both Heck and Suzuki coupling reactions to demonstrate their versatility. Furthermore, the membranes were recovered, reused and shown to be effective for three consecutive coupling reactions.
In addition, and to the best of our knowledge, there are no reports on the fabrication and characterization of Cu2O NP immobilized on electrospun PVP fibrous matrices that have been evaluated as catalytic supports in click chemistry: the Cu(I)-catalysed version of the Huisgen 1,3-dipolar cycloaddition of azides and terminal alkynes, was popularised by Sharpless in 2001 (ref. 31 and 32) and has numerous applications in drug-design and diverse chemical-biology applications.33 Copper34–36 and copper oxide37–39 nanoparticles may act as effective catalysts in click chemistry reactions; consequently, their immobilization on electrospun PVP could extend the usability and flexibility of the click reaction.
Moreover, the chemical compatibility of the PVP–Pd and PVP–Cu2O systems i.e. that the same polymer (PVP) is employed as a stabilizing agent for the generation of NP colloidal solutions and that in both cases the polymer-NP solutions are prepared in methanol, allows for their intermixing. This potentially enables the future development of multicomponent electrospun catalytic supports with an embedded “cocktail” of nanoparticulate catalytic centers.
2. Experimental
2.1. Materials and methods
Polyvinylpyrrolidone (PVP,![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n = 1
n = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), palladium(II) acetate (98%) and copper(II) acetate monohydrate (98% ACS reagent) were purchased from Sigma-Aldrich. Methanol (analytical grade, ACS reagent) was purchased from Scharlau. The above-mentioned reagents were used as provided by the manufacturer without further purification. Benzyl azide was prepared from benzyl bromide and sodium azide according to the literature.40 DMF was dried by azeotropically removing water with benzene and then distilling under vacuum over dried molecular sieves 4 Å and then kept under an argon atmosphere in a desiccator. Anhydrous Na2SO4 was used for drying organic extracts and all volatiles were removed under reduced pressure. All reaction mixtures and column eluents were monitored by TLC using commercial glass backed thin layer chromatography (TLC) plates (Merck Kieselgel 60 F254). The plates were observed under UV light at 254 and 365 nm. The technique of dry flash chromatography was used throughout for all non-TLC scale chromatographic separations using Merck Silica Gel 60 (less than 0.063 mm).41
000), palladium(II) acetate (98%) and copper(II) acetate monohydrate (98% ACS reagent) were purchased from Sigma-Aldrich. Methanol (analytical grade, ACS reagent) was purchased from Scharlau. The above-mentioned reagents were used as provided by the manufacturer without further purification. Benzyl azide was prepared from benzyl bromide and sodium azide according to the literature.40 DMF was dried by azeotropically removing water with benzene and then distilling under vacuum over dried molecular sieves 4 Å and then kept under an argon atmosphere in a desiccator. Anhydrous Na2SO4 was used for drying organic extracts and all volatiles were removed under reduced pressure. All reaction mixtures and column eluents were monitored by TLC using commercial glass backed thin layer chromatography (TLC) plates (Merck Kieselgel 60 F254). The plates were observed under UV light at 254 and 365 nm. The technique of dry flash chromatography was used throughout for all non-TLC scale chromatographic separations using Merck Silica Gel 60 (less than 0.063 mm).41
2.2. Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were prepared as follows:42 in a round bottom flask equipped with a magnetic stirrer, PVP (1.0 g, 9 mmol of vinylpyrridine units) was dissolved in MeOH (10 mL). Subsequently, palladium(II) acetate (100 mg, 0.45 mmol) was added to the polymer solution and the reaction mixture was heated at reflux (65 °C) for 2 h. During this period, the colour of the solution changed from yellow to dark brown indicating the formation of Pd nanoparticles. After the completion of the reaction, the brown-coloured solution was allowed to cool to ca. 20 °C and it was then stored in sealed glass vials. The solutions were stable and no precipitation was observed even after several months.
1) were prepared as follows:42 in a round bottom flask equipped with a magnetic stirrer, PVP (1.0 g, 9 mmol of vinylpyrridine units) was dissolved in MeOH (10 mL). Subsequently, palladium(II) acetate (100 mg, 0.45 mmol) was added to the polymer solution and the reaction mixture was heated at reflux (65 °C) for 2 h. During this period, the colour of the solution changed from yellow to dark brown indicating the formation of Pd nanoparticles. After the completion of the reaction, the brown-coloured solution was allowed to cool to ca. 20 °C and it was then stored in sealed glass vials. The solutions were stable and no precipitation was observed even after several months.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were prepared as follows: in a vial equipped with a magnetic stirrer, PVP (1.0 g, 9 mmol of vinylpyrridine units) was dissolved in MeOH (7.5 mL). Subsequently, copper(II) acetate monohydrate (500 mg, 2.5 mmol) was added to the polymer solution and the mixture was left to stir overnight at ca. 20 °C. During this period, the colour of the solution changed from colourless to blue. Afterwards, hydrazine monohydrate (972 μL, 20.0 mmol) was added and the colour of the solution became dark red, indicating the formation of Cu nanoparticles. Air exposure of the colloidal solution led to a colour change from red to light blue, indicative of the oxidation of the Cu NPs to Cu2O.43 The final PVP-capped Cu2O nanoparticles exhibited high stability in methanol and no agglomeration/destabilization phenomena were observed even after several months.
1) were prepared as follows: in a vial equipped with a magnetic stirrer, PVP (1.0 g, 9 mmol of vinylpyrridine units) was dissolved in MeOH (7.5 mL). Subsequently, copper(II) acetate monohydrate (500 mg, 2.5 mmol) was added to the polymer solution and the mixture was left to stir overnight at ca. 20 °C. During this period, the colour of the solution changed from colourless to blue. Afterwards, hydrazine monohydrate (972 μL, 20.0 mmol) was added and the colour of the solution became dark red, indicating the formation of Cu nanoparticles. Air exposure of the colloidal solution led to a colour change from red to light blue, indicative of the oxidation of the Cu NPs to Cu2O.43 The final PVP-capped Cu2O nanoparticles exhibited high stability in methanol and no agglomeration/destabilization phenomena were observed even after several months.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrospun membrane (11 mg, 2.4 mol%). The mixture was heated to 125 °C in a sealed tube until complete consumption of iodobenzene (TLC). The mixture was then cooled to ca. 20 °C and t-BuOMe (10 mL) was added. The polymer precipitate was filtered and washed with t-BuOMe (10 mL), then dried under vacuum. The membrane was reused in subsequent reactions without other treatment. The organic washings were washed with H2O (2 × 10 mL), combined, dried (Na2SO4) and evaporated to give after column chromatography (n-hexane/DCM, 1
1 electrospun membrane (11 mg, 2.4 mol%). The mixture was heated to 125 °C in a sealed tube until complete consumption of iodobenzene (TLC). The mixture was then cooled to ca. 20 °C and t-BuOMe (10 mL) was added. The polymer precipitate was filtered and washed with t-BuOMe (10 mL), then dried under vacuum. The membrane was reused in subsequent reactions without other treatment. The organic washings were washed with H2O (2 × 10 mL), combined, dried (Na2SO4) and evaporated to give after column chromatography (n-hexane/DCM, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) n-butyl cinnamate (1) (36 mg, 88%) as a yellow oil; Rf 0.72 (n-hexane/DCM, 1
1) n-butyl cinnamate (1) (36 mg, 88%) as a yellow oil; Rf 0.72 (n-hexane/DCM, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); vmax/cm−1 3063w, 3030w, 2958w, 2931w and 2874w (C–H), 1712s (C
1); vmax/cm−1 3063w, 3030w, 2958w, 2931w and 2874w (C–H), 1712s (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1639m (C
O), 1639m (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1578m, 1558m, 1497w, 1465w, 1451m, 1383w, 1327m, 1309m, 1281m, 1256m, 1202m, 1171s, 1119w, 1065w, 1026w, 978m, 864m, 768m; δH(500 MHz; CDCl3) 7.69 (1H, d, J 16.0,
C), 1578m, 1558m, 1497w, 1465w, 1451m, 1383w, 1327m, 1309m, 1281m, 1256m, 1202m, 1171s, 1119w, 1065w, 1026w, 978m, 864m, 768m; δH(500 MHz; CDCl3) 7.69 (1H, d, J 16.0, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.53–7.51 (2H, m, Ar H), 7.39–7.36 (3H, m, Ar H), 6.45 (1H, d, J 16.0,
CH), 7.53–7.51 (2H, m, Ar H), 7.39–7.36 (3H, m, Ar H), 6.45 (1H, d, J 16.0, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 4.21 (2H, t, J 6.7, OCH2), 1.72–1.66 (2H, m, CH2), 1.48–1.40 (2H, m, CH2), 0.97 (3H, t, J 7.4, CH3); δC(125 MHz; CDCl3) 167.05 (s), 144.5 (d), 134.4 (s), 130.15 (d), 128.8 (d), 128.0 (d), 118.2 (d), 64.2 (t), 30.7 (t), 19.15 (t), 13.7 (q); identical to an authentic sample.44
CH), 4.21 (2H, t, J 6.7, OCH2), 1.72–1.66 (2H, m, CH2), 1.48–1.40 (2H, m, CH2), 0.97 (3H, t, J 7.4, CH3); δC(125 MHz; CDCl3) 167.05 (s), 144.5 (d), 134.4 (s), 130.15 (d), 128.8 (d), 128.0 (d), 118.2 (d), 64.2 (t), 30.7 (t), 19.15 (t), 13.7 (q); identical to an authentic sample.44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 membrane (20 mg, 1.6 mol%) and the mixture was heated to ca. 80 °C in a sealed tube until complete consumption of the starting material (TLC). The mixture was then cooled to ca. 20 °C and t-BuOMe (10 mL) was added. The polymer precipitate was filtered and washed with t-BuOMe (10 mL), then dried under vacuum. The membrane was reused in subsequent reactions without further treatment. The organic washings were washed with H2O (2 × 10 mL), combined, dried (Na2SO4) and evaporated to give 4-methoxy-biphenyl (2) (90 mg, 98%) as colourless needles, mp 81–83 °C (from EtOH/H2O, lit.,45 80–82 °C); Rf 0.30 (n-hexane); vmax/cm−1 3067w, 3034w and 3003w (C–H), 1605m, 1582w, 1522m, 1485m, 1464w, 1451w, 1439m, 1287m, 1269m, 1248s, 1200s, 1184m, 1119w, 1042m, 1038m, 1015w, 1003w, 833s, 804, 760s; δH(500 MHz; CDCl3) 7.56 (2H, d, J 7.9, Ar H), 7.54 (2H, d, J 8.7, Ar H), 7.42 (2H, dd, J 7.6, 7.6 Ar H), 7.38 (1H, dd, J 7.4, 7.4 Ar H), 6.99 (2H, d, J 8.7, Ar H), 3.86 (3H, s, CH3); δC(125 MHz; CDCl3) 159.1 (s), 140.8 (s), 133.8 (s), 128.7 (d), 128.1 (d), 126.7 (d), 126.6 (d), 114.2 (d), 55.3 (t); m/z (MALDI-TOF) 185 (MH+, 100%), 170 (32), 152 (61), 141 (18), 130 (32); identical to an authentic sample.
1 membrane (20 mg, 1.6 mol%) and the mixture was heated to ca. 80 °C in a sealed tube until complete consumption of the starting material (TLC). The mixture was then cooled to ca. 20 °C and t-BuOMe (10 mL) was added. The polymer precipitate was filtered and washed with t-BuOMe (10 mL), then dried under vacuum. The membrane was reused in subsequent reactions without further treatment. The organic washings were washed with H2O (2 × 10 mL), combined, dried (Na2SO4) and evaporated to give 4-methoxy-biphenyl (2) (90 mg, 98%) as colourless needles, mp 81–83 °C (from EtOH/H2O, lit.,45 80–82 °C); Rf 0.30 (n-hexane); vmax/cm−1 3067w, 3034w and 3003w (C–H), 1605m, 1582w, 1522m, 1485m, 1464w, 1451w, 1439m, 1287m, 1269m, 1248s, 1200s, 1184m, 1119w, 1042m, 1038m, 1015w, 1003w, 833s, 804, 760s; δH(500 MHz; CDCl3) 7.56 (2H, d, J 7.9, Ar H), 7.54 (2H, d, J 8.7, Ar H), 7.42 (2H, dd, J 7.6, 7.6 Ar H), 7.38 (1H, dd, J 7.4, 7.4 Ar H), 6.99 (2H, d, J 8.7, Ar H), 3.86 (3H, s, CH3); δC(125 MHz; CDCl3) 159.1 (s), 140.8 (s), 133.8 (s), 128.7 (d), 128.1 (d), 126.7 (d), 126.6 (d), 114.2 (d), 55.3 (t); m/z (MALDI-TOF) 185 (MH+, 100%), 170 (32), 152 (61), 141 (18), 130 (32); identical to an authentic sample.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 membrane (11.6 mg, 5.0 mol% Cu). The mixture was heated to ca. 60 °C in a sealed tube until compete consumption of the starting material (TLC). The mixture was then cooled to ca. 20 °C and t-BuOMe (10 mL) was added. The polymer precipitate was filtered and washed with t-BuOMe (10 mL), then dried under vacuum. The membrane was reused in subsequent reactions without further treatment. The organic washings were evaporated to give 1-benzyl-4-phenyl-1H-1,2,3-triazole (3) (102 mg, 87%) as colourless needles, mp 121–123 °C (from c-hexane, lit.,46 123–125 °C); Rf 0.83 (t-BuOMe); vmax/cm−1 3123w, 3098w, 3065w, 3057w, 3036w, 2974w, 2967w, 2928w and 2855 (C–H), 1607w, 1497m, 1483w, 1466m, 1454m, 1443m, 1427w, 1358w, 1225m, 1206m, 1196w, 1182w, 1155w, 1140w, 1076m, 1049m, 1028m, 1003w, 976m, 914m, 827m, 804w, 779m, 766s, 727s; δH(500 MHz; CDCl3) 7.80 (2H, d, J 7.3, Ar H), 7.68 (1H, s, Ar H), 7.41–7.35 (5H, m, Ar H), 7.32–7.29 (3H, m, Ar H), 5.55 (2H, s, CH2); δC(125 MHz; CDCl3) 148.1 (s), 134.6 (s), 130.45 (s), 129.0 (d), 128.71 (d), 128.66 (d), 128.1 (d), 128.0 (d), 125.6 (d), 119.5 (d), 54.1 (t); m/z (MALDI-TOF) 236 (MH+, 100%), 192 (40), 181 (18), 166 (37); identical to an authentic sample.
1 membrane (11.6 mg, 5.0 mol% Cu). The mixture was heated to ca. 60 °C in a sealed tube until compete consumption of the starting material (TLC). The mixture was then cooled to ca. 20 °C and t-BuOMe (10 mL) was added. The polymer precipitate was filtered and washed with t-BuOMe (10 mL), then dried under vacuum. The membrane was reused in subsequent reactions without further treatment. The organic washings were evaporated to give 1-benzyl-4-phenyl-1H-1,2,3-triazole (3) (102 mg, 87%) as colourless needles, mp 121–123 °C (from c-hexane, lit.,46 123–125 °C); Rf 0.83 (t-BuOMe); vmax/cm−1 3123w, 3098w, 3065w, 3057w, 3036w, 2974w, 2967w, 2928w and 2855 (C–H), 1607w, 1497m, 1483w, 1466m, 1454m, 1443m, 1427w, 1358w, 1225m, 1206m, 1196w, 1182w, 1155w, 1140w, 1076m, 1049m, 1028m, 1003w, 976m, 914m, 827m, 804w, 779m, 766s, 727s; δH(500 MHz; CDCl3) 7.80 (2H, d, J 7.3, Ar H), 7.68 (1H, s, Ar H), 7.41–7.35 (5H, m, Ar H), 7.32–7.29 (3H, m, Ar H), 5.55 (2H, s, CH2); δC(125 MHz; CDCl3) 148.1 (s), 134.6 (s), 130.45 (s), 129.0 (d), 128.71 (d), 128.66 (d), 128.1 (d), 128.0 (d), 125.6 (d), 119.5 (d), 54.1 (t); m/z (MALDI-TOF) 236 (MH+, 100%), 192 (40), 181 (18), 166 (37); identical to an authentic sample.2.3. Characterisation methods
The UV-vis spectra of the PVP–Pd and the PVP–Cu nanohybrids stabilized in MeOH were recorded on a Jasco V-630 UV-vis spectrophotometer operating at ca. 20 °C, after appropriate dilution of the as-prepared colloidal solutions.High resolution transmission electron microscopy (HRTEM) investigations of the membranes were performed by using a TECNAI F30 G2 S-TWIN microscope operated at 300 kV equipped with energy dispersive X-ray spectrometer (EDX). Samples were placed into a double copper grid (oyster) to be visualized by TEM. The morphology of the nanocomposite membranes was also characterised by scanning electron microscopy (SEM) (Vega TS5136LS-Tescan). The samples were gold-sputtered (∼15 nm) (sputtering system K575X Turbo Sputter Coater – Emitech) prior to SEM inspection.
For the characterisation of the final products obtained from the catalytic reactions: Melting points were determined using a PolyTherm-A, Wagner & Munz, Koefler – Hotstage Microscope apparatus. IR spectra were recorded on a Shimadzu FTIR-NIR Prestige-21 spectrometer with Pike Miracle Ge ATR accessory and strong, medium and weak peaks are represented by s, m and w, respectively. 1H and 13C NMR spectra were recorded on a Bruker Avance 500 machine (at 500 and 125 MHz, respectively). Deuterated solvents were used for homonuclear lock and the signals are referenced to the deuterated solvent peaks. CH assignments are made based on DEPT 135 spectroscopy. MALDI-TOF mass spectra were recorded on a Bruker Autoflex III Smartbeam instrument.
3. Results and discussion
3.1. PVP–Pd, PVP–Cu and PVP–Cu2O colloidal solutions
The PVP-coated Pd and Cu MNPs were prepared in the form of stable colloidal solutions in MeOH according to the literature.42,47 The reaction process involved the reduction of Pd(II) ions into metallic Pd(0) NPs in refluxing MeOH that functioned as both, solvent and reductant in the presence of PVP which acted as a steric stabilizer. In the case of the Cu(II) ions, hydrazine monohydrate was used as the reductant for generating metallic PVP-capped Cu(0) NPs (Fig. 1). | ||
| Fig. 1 Schematic presentation of the synthetic pathways followed for the generation of the PVP–Pd, PVP–Cu and PVP–Cu2O colloidal solutions stabilized in MeOH and corresponding photographs. | ||
The PVP, PVP–Pd and PVP–Cu MeOH solutions were characterised by UV-vis spectroscopy (Fig. 2). The broad absorption tail appearing between 300–800 nm is typical for Pd MNP colloidal systems,48,49 whereas in the case of the Cu-containing solutions the characteristic surface plasmon resonance (SPR) signal appears at around 580 nm, indicating the existence of Cu MNP in solution.50 When the freshly prepared PVP–Cu colloidal solution was exposed to air, the colour of the solution gradually changed from red to light blue (Fig. 1), indicating the oxidation of the Cu NP to Cu2O NP. Similar solution colour changes (i.e. from red to light blue) were previously reported for oleylamine-coated Cu and Cu2O NPs.43
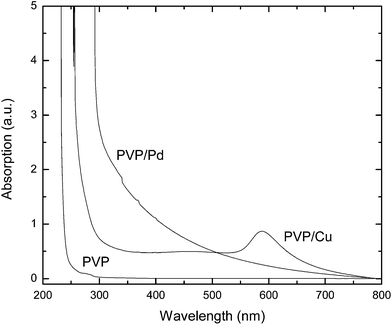 | ||
| Fig. 2 UV-vis spectra of the pristine PVP MeOH solution and the PVP–Pd and PVP–Cu hybrid colloidal solutions (recorded upon appropriate dilution of the as-prepared colloidal solutions). | ||
3.2. Membrane fabrication
Fibrous membranes comprised of PVP and either Pd or Cu2O nanoparticles were prepared by electrospinning (Fig. 3).By systematically varying the solution concentration, the applied voltage, the delivery rate of the solution, the diameter of the needle and the needle-to-collector distance, the optimum experimental parameters were determined for the production of fibrous PVP–Pd (Table 1, entry 1) and PVP–Cu2O (Table 1, entry 2) electrospun membranes.
| Solutions concentration | |||
|---|---|---|---|
| Entry | Sample code | Solution concentration (% w/v) | Moles of VP units to metal salt ratio |
| 1 | PVP | 15 | — |
| 2 | PVP–Pd | 10 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | PVP–Cu2O | 10 | 3.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Electrospinning conditions | ||||
|---|---|---|---|---|
| Entry | Needle (G) | Voltage (KV) | Needle-to-collector distance (cm) | Flow rate (μL min−1) |
| 1 | 18 | 20 | 20 | 3.0 |
| 2 | 16 | 15 | 20 | 5.0 |
| 3 | 16 | 20 | 10 | 2.5 |
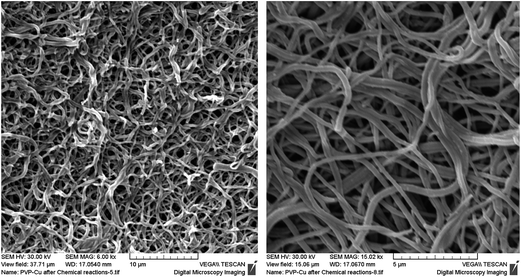
The morphology of the membranes was studied using SEM and TEM. As seen in the SEM images (Fig. 4), in all cases continuous, bead-free cylindrical fibers were obtained under the optimum electrospinning conditions employed. The average diameters of the PVP, PVP–Pd and PVP–Cu2O fibers were 9.36 ± 1.68, 8.64 ± 2.98 and 7.11 ± 1.04 μm, respectively; determined using digimizer image analysis software. Moreover, no significant changes were observed in the morphology of the fibers in the presence of the embedded Pd and Cu2O nanoparticles.
 | ||
| Fig. 4 SEM images of (a) the pristine PVP, (b) the PVP–Pd and (c) the PVP–Cu2O nanocomposite electrospun membranes. | ||
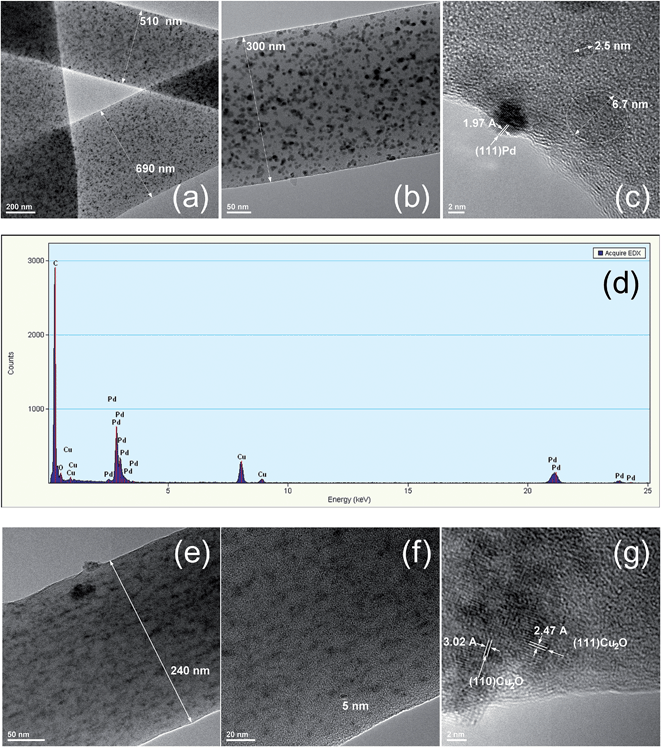
The as-prepared Pd and Cu2O containing fibrous nanocomposites were also visualized by TEM (Fig. 5). From the TEM bright field images (Fig. 5a, b, d–f) the inorganic nanoparticles embedded in the membranes appear spherical in shape with average diameters of ∼5 nm. Moreover, a distinct dispersion of the NP in the PVP fibrous matrix resulting in high homogeneity was observed in both cases. HRTEM analysis was only feasible for the PVP–Pd membranes since the PVP–Cu2O system was unstable in the electron beam. The Pd nanoparticles embedded in the fibrous PVP matrix are nanocrystals and HRTEM imaging (Fig. 5c) discloses the crystalline planes (111) and (200) of Pd NP with characteristic interplanar distances of 2.27 and 1.97 Å, respectively. In addition, the EDX spectrum corresponding to the PVP–Pd system (Fig. 5g) shows the presence of Pd and O as the major elements in the sample. The presence of Cu is attributed to the Cu grid employed in TEM investigations. In the case of the PVP–Cu2O, the EDX analysis could not provide useful information since a Cu grid was employed during the sample analysis and the expected oxygen exists both in the polymer matrix and in the Cu2O nanoparticles.
Thermal crosslinking of the membranes was carried out to prevent membrane dissolution and NP leaching during the use of the aforementioned materials as heterogeneous catalytic supports in the organic reaction media. The morphology and compositional characteristics of the nanocomposite fibers after thermal treatment were determined by means of SEM and TEM/EDX analysis.
SEM images of the thermally-induced crosslinked PVP–Pd and PVP–Cu2O electrospun nanocomposite membranes (Fig. 6a and b) indicated that the membranes maintained their cylindrical shape and that there were no significant morphology changes compared to the non-crosslinked systems. After thermal treatment, the membranes were immersed in an aqueous solution where no membrane dissolution was observed (as in the case of the non-crosslinked analogues that are water-soluble due to the hydrophilic character of PVP) – Fig. 6c – indicating that the crosslinking process was successful.
In Fig. 7 the corresponding TEM images accompanied by EDX analysis spectra are provided. Owing to the superior stability of the crosslinked vs. non-crosslinked materials, it was possible to perform HRTEM analysis on both systems and confirm the existence of Cu2O NP in the PVP–Cu2O membranes. As in the case of the non-crosslinked PVP–Pd electrospun membranes, spherical Pd nanocrystals embedded in the crosslinked PVP matrix can be visualized in the bright field TEM images (Fig. 7a and b) while HRTEM disclosed the characteristic crystalline planes of Pd NP (Fig. 7c). The presence of the Pd NP in the crosslinked membranes was also verified by EDX analysis (Fig. 7d). HRTEM confirmed the existence of Cu2O NP in the PVP–Cu2O crosslinked membranes (Fig. 7g) since the characteristic crystalline planes (110) and (111) of Cu2O NP with respective 3.02 and 3.47 Å interplanar distances can be clearly seen.
3.3. Catalysis
Having in our hands both a crosslinked and a non-crosslinked membrane containing Pd nanoparticles, we performed the same Heck reaction, using 1.5 equiv. of the acrylate, 2.7 equiv. of Et3N and 7.4 mol% Pd from both the crosslinked and non-crosslinked material. After 14 h at ca. 125 °C, regardless of which catalyst was used, the starting iodide was completely consumed and product 1 was formed in 88% isolated yield (Scheme 1). Under these conditions the non-crosslinked membrane dissolved to give, as expected, a dark brown coloured solution, while the crosslinked membrane was more tolerant to the reaction conditions (DMF, 125 °C, 14 h). Nevertheless, attempts to recover the polymer catalysts after the reactions had completed by filtration led to low recoveries of up to ∼60%.
Reducing the catalyst loading and improving its post reaction recovery would prove the usability of the method. Using a lower loading of Pd (2.4 mol%) and lowering the reaction temperature to 100 °C with both the crosslinked and the non-crosslinked polymer systems, successfully gave the desired product in good yields (Table 2). Furthermore, modifying the reaction work-up allowed the recovery and reuse of the colloidal PVP–Pd nanocatalyst: the reaction was performed in a sealed tube to avoid the loss of volatile reagents and after the end of the reaction t-BuOMe was added to precipitate the PVP–Pd nanocomposite. The remaining solution was then decanted, washed with water to remove the DMF and chromatographed to isolate the pure product. In this manner the precipitated polymer-based catalyst can be reused without further processing. This procedure was repeated up to three times to demonstrate the reusability of the catalysts and examine whether there were any notable differences between the two catalytic systems. Gratifyingly, both catalysts were active even during the 3rd run and gave consistent high yields of product.
The reactions involving the crosslinked material showed a marked drop in product yield (from 97% in run 1 to 88 and 89% in runs 2 and 3), however, a similar drop was not observed with the non-crosslinked membrane. This phenomenon may be attributed to partial degradation of the membrane morphology under the reaction conditions that reduces its active surface area and consequently decreased its catalytic efficacy. To verify this hypothesis SEM analysis was performed on the crosslinked PVP–Pd membrane recovered after three consecutive reaction runs. Indeed, as seen in the SEM images (Fig. 8b), cylindrical fibers with larger mean diameters compared to the as-prepared crosslinked analogue (Fig. 8a) are obtained after the third reaction run. The above-mentioned problem may be overcome by performing the Heck reaction in different organic solvents or under milder reaction conditions.
The click reaction between benzyl azide and phenylacetylene in the presence of Et3N and PVP–Cu2O nanoparticles was completed in 2 h and gave 1-benzyl-4-phenyl-1H-1,2,3-triazole (3) in 87% yield (Scheme 3). The polymer catalyst was reused in three consecutive reaction runs, and a small drop in product yield indicated a gradual loss of catalyst reactivity. SEM characterisation of the crosslinked PVP–Cu2O membranes recovered after three runs revealed that although the fibers retain their cylindrical shape they had begun to aggregate (Fig. 9), similar to the above results on the PVP–Pd crosslinked membrane employed in Heck reaction processes.
 | ||
| Scheme 3 Click reaction between benzyl azide and phenylacetylene in the presence of the crosslinked PVP–Cu2O membrane. TON: turnover number. | ||
4. Conclusions
Catalytic Pd and Cu2O NP have been immobilized in electrospun PVP crosslinked mats and the resulting fibrous nanocomposites evaluated as heterogeneous catalytic supports in Heck, Suzuki (PVP–Pd) and click chemistry (PVP–Cu2O) reactions. By employing a new recovery protocol, the crosslinked membranes can be recycled and exhibit high catalytic efficiencies even after three consecutive reaction runs. The versatility of PVP to act as effective steric stabiliser for various nanoparticles in organic solvents (including Ag, Au, Cu, Pd, Cu2O etc.) allowing for their intermixing within a single electrospun fibrous mat, creates new prospects for the future development of multifunctional electrospun catalytic supports.Acknowledgements
The authors wish to thank the Cyprus Research Promotion Foundation (Grant no. NEAYPODOMH/NEKYP/0308/02 and NEAYPODOMH/STRATHII/0308/06) and the following organizations and companies in Cyprus for generous donations of chemicals and glassware: the State General Laboratory; the Agricultural Research Institute; the Ministry of Agriculture; MedoChemie Ltd; Medisell Ltd; and Biotronics Ltd. The University of Cyprus and the Program “New Researchers” supporting P. Papaphilippou is greatly acknowledged. Furthermore, we thank the A. G. Leventis Foundation for helping to establish the NMR facility at the University of Cyprus.References
- Z. M. Huang, Y. Z. Zhang, M. Kotaki and S. Ramakrishna, Compos. Sci. Technol., 2003, 63, 2223 CrossRef CAS.
- J. Venugopal, Y. Z. Zhang and S. Ramakrishna, Proc. Inst. Mech. Eng., Part N, 2005, 218, 35 Search PubMed.
- D. H. Reneker, A. L. Yarin, E. Zussman and H. Xu, Adv. Appl. Mech., 2007, 41, 43 Search PubMed.
- T. Subbiah, G. S. Bhat, R. W. Tock, S. Parameswaran and S. S. Ramkumar, J. Appl. Polym. Sci., 2005, 96, 557 CrossRef CAS.
- J. Doshi and D. H. Reneker, J. Electrost., 1995, 35, 151 CrossRef CAS.
- D. H. Reneker and I. Chun, Nanotechnology, 1996, 7, 216 CrossRef CAS.
- H. Fong, I. Chun and D. H. Reneker, Polymer, 1999, 40, 4585 CrossRef CAS.
- S. Agarwal, A. Greiner and J. H. Wendorff, Prog. Polym. Sci., 2013, 38, 963 CrossRef CAS PubMed.
- S. Wang, Z. Wang and Z. Zha, Dalton Trans., 2009, 9363 RSC.
- B. R. Cuenya, Thin Solid Films, 2010, 518, 3127 CrossRef CAS PubMed.
- N. N. Nassar, A. Hassan, L. Carbognani, F. Lopez-Linares and P. Pereira-Almao, Fuel, 2012, 95, 257 CrossRef CAS PubMed.
- A. Cao, R. Lu and G. Veser, Phys. Chem. Chem. Phys., 2010, 12, 13499 RSC.
- M. Zahmakiran and S. Ozkar, Nanoscale, 2011, 3, 3462 RSC.
- R. Narayanan and M. A. El-Sayed, J. Phys. Chem. B, 2005, 109, 12663 CrossRef CAS PubMed.
- L. Guo, J. Bai, C. Li, Q. Meng, H. Liang, W. Sun, H. Li and H. Liu, Appl. Surf. Sci., 2013, 283, 107 CrossRef CAS PubMed.
- N. Guang-Di, L. Shang-Kun, L. Xiao-Feng and W. Ce, Chem. Res. Chin. Univ., 2013, 34, 15 Search PubMed.
- N. A. M. Barakat, Appl. Catal., A, 2013, 451, 21 CrossRef CAS PubMed.
- Z. G. Zhao, Z. J. Yao, J. Zhang, R. Zhu, Y. Jin and Q. W. Li, J. Mater. Chem., 2012, 22, 16514 RSC.
- Z. Zhang, C. Shao, Y. Sun, J. Mu, M. Zhang, P. Zhang, Z. Guo, P. Liang, C. Wang and Y. Liu, J. Mater. Chem., 2012, 22, 1387 RSC.
- R. Riz-Rosas, J. Bedia, J. M. Rosas, M. Lallave, I. G. Loscertales, J. Rodriguez-Mirasol and T. Cordero, Catal. Today, 2012, 187, 77 CrossRef PubMed.
- M. Shang, W. Wang, S. Sun, E. Gao, Z. Zhang, L. Zhang and R. O'Hayre, Nanoscale, 2013, 5, 5036 RSC.
- L. Zhang, A. Aboagye, A. Kelkar, C. Lai and H. Fong, J. Mater. Sci., 2014, 49, 463 CrossRef CAS.
- S. Xiao, M. Shen, R. Guo, Q. Huang, S. Wang and X. Shi, J. Mater. Chem., 2010, 20, 5700 RSC.
- Z. G. Wang, B. B. Ke and Z. K. Xu, Biotechnol. Bioeng., 2007, 97, 708 CrossRef CAS PubMed.
- L. Guo, J. Bai, H. Liang, T. Xu, C. Li, Q. Meng, H. Liu and Y. Huang, Korean J. Chem. Eng., 2013, 30, 2142 CrossRef CAS.
- L. Gardella, A. Basso, M. Prato and O. Monticelli, ACS Appl. Mater. Interfaces, 2013, 5, 7688 CAS.
- M. M. Demir, M. A. Gulgun, Y. Z. Mencelogu, B. Erman, S. S. Abramchuk, E. E. Makhaeva, A. R. Khokhlov, V. G. Matveeva and M. G. Sulman, Macromolecules, 2004, 37, 1787 CrossRef CAS.
- S. Xiao, W. Xu, H. Ma and X. Fang, RSC Adv., 2012, 2, 319 RSC.
- M. Chen, C. Wang, W. Fang, J. Wang, W. Zhang, G. Jin and G. Diao, Langmuir, 2013, 29, 11858 CrossRef CAS PubMed.
- T. G. Kim, D. Ragupathy, A. Iyengar Gopalan and K. P. Lee, Nanotechnology, 2010, 21, 134021 CrossRef PubMed.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS.
- P. Thirumurugan, D. Matosiuk and K. Jozwiak, Chem. Rev., 2013, 113, 4905 CrossRef CAS PubMed.
- B. H. Lipshutz and B. R. Taft, Angew. Chem., Int. Ed., 2006, 45, 8235 CrossRef CAS PubMed.
- A. Dhakshinamoorthy, S. Navalon, D. Sempere, M. Alvaro and H. Garcia, Chem. Commun., 2013, 49, 2359 RSC.
- E. A. Karakhanov, A. L. Maximov, Y. S. Kardasheva, V. A. Skorkin, S. V. Kardashev, V. V. Predeina, M. Y. Talanova, E. Lurie-Luke, J. A. Seeley and S. L. Cron, Appl. Catal., A, 2010, 385, 62 CrossRef CAS PubMed.
- V. P. Reddy, A. V. Kumar, K. Swapna and K. R. Rao, Org. Lett., 2009, 11, 951 CrossRef CAS PubMed.
- A. Kamal, V. Srinivasulu, J. N. S. R. C. Murty, N. Shankaraiah, N. Nagesh, T. Srinivasa Reddy and A. V. Subba Rao, Adv. Synth. Catal., 2013, 355, 2297 CrossRef CAS.
- A. R. Rosario, K. K. Casola, C. E. S. Oliveira and G. Zeni, Adv. Synth. Catal., 2013, 355, 2960 CrossRef CAS.
- L. Campbell-Verduyna, P. H. Elsinga, L. Mirfeizi, R. A. Dierckx and B. L. Feringa, Org. Biomol. Chem., 2008, 6, 3461 Search PubMed.
- L. M. Harwood, Aldrichimica Acta, 1985, 18, 25 Search PubMed.
- J. S. Bradley, E. W. Hill, S. Behal, C. Klein, A. Duteil and B. Chaudret, Chem. Mater., 1992, 4, 1234 CrossRef CAS.
- M. Salavati-Niasari and F. Davar, Mater. Lett., 2009, 63, 441 CrossRef CAS PubMed.
- T. Mino, M. Shibuya, S. Suzuki, K. Hirai, M. Sakamoto and T. Fujita, Tetrahedron, 2012, 68, 429 CrossRef CAS PubMed.
- N. R. Guha, C. B. Reddy, N. Aggarwal, D. Sharma, A. K. Shil and B. P. Das, Adv. Synth. Catal., 2012, 354, 2911 CrossRef CAS.
- H. A. Stefani, S. N. S. Vasconcelos, F. Manarin, D. M. Leal, F. B. Souza, L. S. Madureira, J. Zukerman-Schpector, M. N. Eberlin, M. N. Godoi and R. S. Galaverna, Eur. J. Org. Chem., 2013, 3780 CrossRef CAS.
- A. Sarkar, T. Mukherjee and S. Kapoor, J. Phys. Chem. C, 2008, 112, 3334 CAS.
- I. Papagiannouli, M. Demetriou, S. Couris and T. Krasia-Christoforou, RSC Adv., 2014, 4, 8779 RSC.
- I. Papagiannouli, M. Demetriou, G. Chatzikyriakos, K. Iliopoulos, T. Krasia-Christoforou and S. Couris, Opt. Mater., 2014, 36, 123 CrossRef PubMed.
- S. Venkatakrishnan, G. Veerappan, E. Elamparuthi and A. Veerappan, RSC Adv., 2014, 4, 15003 RSC.
- F. Lu, J. Ruiz and D. Astruc, Tetrahedron Lett., 2004, 45, 9443 CrossRef CAS PubMed.
- K. B. Sharpless, V. V. Fokin, V. V. Rostovtsev, L. Noodleman, R. Hilgraf, T. Lovell and F. Himo, J. Am. Chem. Soc., 2005, 127, 210 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2014 |