Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Suzuki homo-coupling reaction based fluorescent sensors for monosaccharides†
Su-Ying Xu*a,
Hui-Chen Wanga,
Stephen E. Flowera,
John S. Fosseyb,
Yun-Bao Jiang*c and
Tony D. James*a
aDepartment of Chemistry, University of Bath, Bath BA2 7AY, UK. E-mail: suying.xu@bath.edu; t.d.james@bath.ac.uk
bThe School of Chemistry, The University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK
cDepartment of Chemistry, College of Chemistry and Chemical Engineering, and the MOE Key Laboratory of Spectrochemical Analysis and Instrumentation, Xiamen University, Xiamen 361005, China. E-mail: ybjiang@xmu.edu.cn
First published on 30th July 2014
Abstract
Palladium catalysed, aryl boronic acid homocoupling, is explored as a fluorescence sensing regime for saccharides. The catalytic formation rate of fluorescent bi-aryls, under control of a palladium catalyst, is modulated by the presence of saccharides. The nature of the aryl group, rate of biaryl formation and limits of detection are investigated.
Introduction
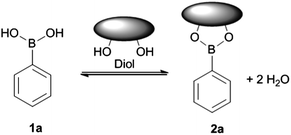
Saccharide recognition is vital to the development and survival of life and has attracted significant interest.1–5 However, the development of synthetic, saccharide recognition domains that can compete with bulk water to recognise these heavily solvated saccharide molecules remains a challenging problem.6–8 Aryl boronic acids reversibly bind covalently with 1,2- and 1,3-diols to form five- and six-member boronate esters, respectively (Fig. 1).The reversible covalent binding of boronic acids with saccharides has been extensively explored in the area of direct saccharide sensing.5,9–11 Colorimetric and fluorometric boronic acid based saccharide sensors rely on a linear response generated by saccharides interacting with a defined stoichiometry of boronic acid sensor, to elicit the colour or fluorescence responses they display. The synthesis of new sensor systems can often be challenging yet the search for more sensitive sensors remains a highly active area of research. Indicator displacement assays partially relieve synthetic effort since the signal reporter moiety does not need to be directly linked to the binding unit (boronic acid moiety).12–14 Using a similar concept a boronic acid-modified quencher was used by Singaram et al.,15 in this system binding between boronic acid (quencher) and saccharide released an anionic dye, resulting in signal recovery. While saccharide selectivity often requires multiple boronic acid receptors, Jiang and co-workers have developed a series of glucose-selective monoboronic acid derivatives using the non-covalent “linking” of boronic acid moieties to afford optimal binding conformation for D-glucose.16,17 However, the reversible interaction between a boronic acid and diols typically allow binding of sugar at mM or sub-mM levels. Thus a high concentration of chemosensor is required to generate a detectable signal.
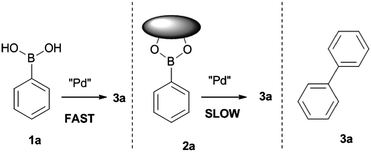
New chemosensing strategies with enhanced sensitivity are in great demand. In previous work by some of the authors included in this report, the palladium catalysed formation of biphenyl via a homocoupling of phenylboronic acid was used as a probe to detect saccharide binding.18 The approach relies on the catalytic formation of the reporter molecule, therefore the sensitivity is not limited to the detection of the added probe rather the formation of biphenyl giving enhanced opportunities for sensitivity in colorimetric and fluorometric sensing, since the background and noise are minimised. In this system it is the rate of probe formation that gives information on the concentration of the saccharide analyte.
In a palladium catalysed Suzuki homo-coupling reaction the nature of the boron species affects the rate of reaction. Boronic esters react more slowly than boronic acids in these reactions; therefore the formation of a boronic ester with a saccharide slows bi-aryl formation. The boronic ester formed between an aryl boronic acid moiety and probe saccharides slows the rate of reaction towards Suzuki homo-coupling.
In the earlier work with phenylboronic acid (1a) as the substrate for catalytic saccharide sensing, the product biphenyl has excitation and emission wavelengths of 250 nm and 311 nm, respectively. These wavelengths are sub-optimal for practical sensing applications, since exogenous fluorophores could interfere and impair the accuracy of this sensing system. As such alternative boronic acid substrates are required in order to deliver output molecules with longer wavelength excitation and emission profiles to avoid interference from exogenous fluorophores.
Results and discussion
In addition to the previously tested phenyl boronic acid (1a), naphthalene-1-boronic acid (1b) and 1,4-phenyldiboronic acid (1c), (Scheme 1), were selected as substrates for comparison in the catalytic Suzuki homo-coupling based saccharide sensing strategy (Fig. 2).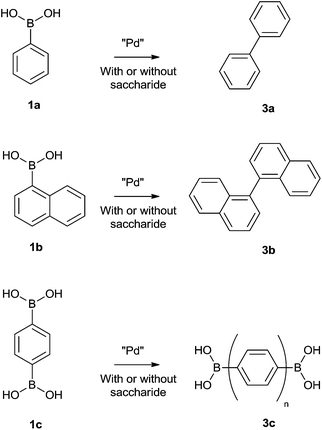 | ||
| Scheme 1 Structures of phenylboronic acid (1a), naphthalene-1-boronic acid (1b) and 1,4-phenyldiboronic acid (1c) and their corresponding homocoupling products (3a, 3b and 3c respectively). | ||
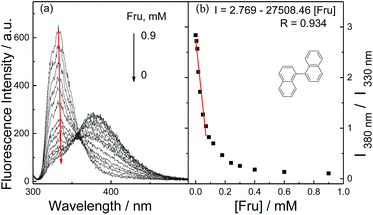
For naphthalene-1-boronic acid (1b) when the catalyst was added, a new emission at 380 nm was observed along with the disappearance of its intrinsic emission at 330 nm (S1†), which was a very encouraging result since as well as extending the wavelength, the system is now a ratiometric fluorescence sensing platform for saccharides. The reaction was monitored using TLC and the binaphthyl product was verified using an authentic sample of 1,1′-binaphthyl (S2†). The optimum time for each measurement to obtain the maximum difference between the catalysed reaction with and without fructose was 25 minutes (S3†). The titration of 1b (Fig. 3) with D-fructose revealed that 1 mM of D-fructose (4 mM in the case of the PBA system, shown in S4†) was required to fully quench the reaction, demonstrating that the sensitivity of the catalytic sensing strategy can be modified by changing substrates. More importantly the ratiometric sensing observed could eliminate possible interference in the system and also lead to a calibration free system. Using the same protocol, saccharide titrations with D-galactose and D-glucose were also investigated and the usual saccharide order of affinities towards the boronic acid moiety was observed (Table 1).19 The limit of detection (LOD) indicates that an improved level of sensitivity was observed using the naphthalene-1-boronic acid, compared with our previous work. The improved sensitivity might be attributed to the establishment of the internal calibration of this sensing system.
| Limit of detection, mol L−1 | |||
|---|---|---|---|
| D-Fructose | D-Galactose | D-Glucose | |
| 1a (I311 nm) | 2.4 × 10−5 | 3.3 × 10−4 | 6.1 × 10−3 |
| 1b (I380 nm/I330 nm) | 1.8 × 10−5 | 2.0 × 10−4 | 1.2 × 10−3 |
1,4-Phenydiboronic acid (1c) was also utilised under the same conditions; in this system the fluorescent intensity displayed an initial increase, but the intensity reduced over time. We attributed this to poor water solubility of the resulting polymer product, i.e. the fluorescence decreases as the product desolvates. However, a good linear relationship between fluorescent emission and the concentration of saccharide is obtained before the fluorescence intensity decreases. The D-fructose titration was carried out by collecting fluorescent spectra after six minutes at different concentrations of saccharides. The 1,4-phenyldiboronic acid displayed a red-shifted maximum emission and quicker response, which might be due to the extended conjugation of the generated products and also the reactivity of the substrate towards Suzuki homo-coupling reactions (Fig. 4).
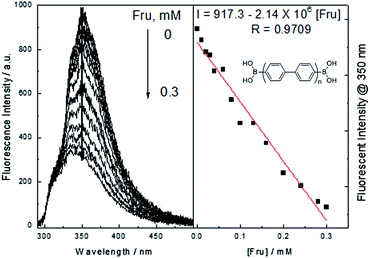 | ||
| Fig. 4 Change of the fluorescent intensity at 350 nm with different concentration of fructose after six minutes under the catalysis of palladium in buffer solution = 5 × 10−6 M, λex = 280 nm. | ||
It is worth noting that all the substrates were investigated under the same experimental conditions as the previous work without further optimisation toward each substrate for easier comparison. Given the popularity of the Suzuki coupling reaction and the multitude of boronic acid derivatives that are readily available to use, saccharide recognition/sensing based on the Suzuki homo-coupling reaction could be easily tuned through the variation of substrates to afford practically applicable saccharide selective sensing platforms.
Conclusions
The magnitude of inhibition for all the boronic acids investigated 1a, 1b and 1c follow the same order as the affinities of saccharides toward the boronic acid moiety.19 This demonstrates that the Suzuki homo-coupling reaction can be used as a general platform for the sensing of saccharides. More interestingly boronic acid 1b affords a ratiometric chemosensor with improved sensitivity and di-boronic acid 1c displays faster response and an improved linear relationship with increasing concentrations of saccharide. Given the multitude of boronic acids, our results indicate that it should be possible to extend this sensing platform and develop application specific chemosensors, with appropriate sensitivity and response time to match any desired saccharide sensing requirements.Experimental section
Reagents and solvents were purchased from Sigma-Aldrich and Fisher, and were used directly without further purification. Fluorescence spectra were recorded on a Perkin Elmer Luminescence Spectrophotometer LHB50 fluorescence spectrometer using a 1 cm quartz cell. Buffer solutions were 0.02 M NaHCO3–Na2CO3, calibrated by Hanna Instruments HI 9321 Microprocessor pH meter.NaHCO3–Na2CO3 buffer solution was made up by dissolving 0.53 g Na2CO3 and 1.68 g NaHCO3 into 250 mL deionised water (≥18.2 MΩ), with a pH value of 9.3. The stock solution of palladium catalyst was 1.0 × 10−3 M, made from PdCl2 dissolved in MeOH. All the experiments were carried out at room temperature.
Acknowledgements
TDJ and SYX are grateful for financial support from China Scholarship Council (CSC) and University of Bath for a Full Fees Scholarship. TDJ, JSF, YBJ and SYX thank the Royal Society-NSFC International Exchanges Scheme: China cost-share programme for funding exchanges between the UK and China. The Catalysis And Sensing for our Environment (CASE) network is thanked for promoting research exchange opportunities. TDJ thanks Xiamen University for a guest professorship.Notes and references
- T. D. James, M. D. Phillips and S. Shinkai, Boronic acids in saccharide recognition, Royal Society of Chemistry, 2006 Search PubMed.
- M. S. Steiner, A. Duerkop and O. S. Wolfbeis, Chem. Soc. Rev., 2011, 40, 4805–4839 RSC.
- H. S. Mader and O. S. Wolfbeis, Microchim. Acta, 2008, 162, 1–34 CrossRef CAS.
- T. D. James, in Creative Chemical Sensor Systems, ed. T. Schrader, Springer-Verlag Berlin, Berlin, 2007, vol. 277, pp. 107–152 Search PubMed.
- R. Nishiyabu, Y. Kubo, T. D. James and J. S. Fossey, Chem. Commun., 2011, 47, 1106–1123 RSC.
- D. B. Walker, G. Joshi and A. P. Davis, Cell. Mol. Life Sci., 2009, 66, 3177–3191 CrossRef CAS PubMed.
- E. Klein, Y. Ferrand, N. P. Barwell and A. P. Davis, Angew. Chem., Int. Ed., 2008, 47, 2693–2696 CrossRef CAS PubMed.
- A. P. Davis and R. S. Wareham, Angew. Chem., Int. Ed., 1999, 38, 2978–2996 CrossRef.
- S. D. Bull, M. G. Davidson, J. M. H. van den Elsen, J. S. Fossey, A. T. A. Jenkins, Y.-B. Jiang, Y. Kubo, F. Marken, K. Sakurai, J. Zhao and T. D. James, Acc. Chem. Res., 2013, 46, 312–326 CrossRef CAS PubMed.
- X. Wu, Z. Li, X.-X. Chen, J. S. Fossey, T. D. James and Y.-B. Jiang, Chem. Soc. Rev., 2013, 42, 8032–8048 RSC.
- J. S. Fossey, F. D'Hooge, J. M. H. van den Elsen, M. P. P. Morais, S. I. Pascu, S. D. Bull, F. Marken, A. T. A. Jenkins, Y.-B. Jiang and T. D. James, Chem. Rec., 2012, 12, 464–478 CrossRef CAS PubMed.
- G. Springsteen and B. Wang, Chem. Commun., 2001, 1608–1609 RSC.
- S. Arimori, C. J. Ward and T. D. James, Tetrahedron Lett., 2002, 43, 303–305 CrossRef CAS.
- V. Janowski and K. Severin, Chem. Commun., 2011, 47, 8521–8523 RSC.
- D. B. Cordes, A. Miller, S. Gamsey, Z. Sharrett, P. Thoniyot, R. Wessling and B. Singaram, Org. Biomol. Chem., 2005, 3, 1708–1713 CAS.
- X. Wu, L.-R. Lin, Y.-J. Huang, Z. Li and Y.-B. Jiang, Chem. Commun., 2012, 48, 4362–4364 Search PubMed.
- Y.-J. Huang, W.-J. Ouyang, X. Wu, Z. Li, J. S. Fossey, T. D. James and Y.-B. Jiang, J. Am. Chem. Soc., 2013, 135, 1700–1703 Search PubMed.
- S.-Y. Xu, Y.-B. Ruan, X.-X. Luo, Y.-F. Gao, J.-S. Zhao, J.-S. Shen and Y.-B. Jiang, Chem. Commun., 2010, 46, 5864–5866 Search PubMed.
- J. P. Lorand and J. O. Edwards, J. Org. Chem., 1959, 24, 769–774 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07331b |
| This journal is © The Royal Society of Chemistry 2014 |