Synthesis of dibenzoxanthene and acridine derivatives catalyzed by 1,3-disulfonic acid imidazolium carboxylate ionic liquids†
Arup Kumar Dutta,
Pinky Gogoi and
Ruli Borah*
Department of Chemical Sciences, Tezpur University, Napaam, Tezpur, Assam 784028, India. E-mail: ruli@tezu.ernet.in
First published on 27th August 2014
Abstract
New members of 1,3-disulfonic acid imidazolium carboxylate ionic liquids [DSIM][X] (where X = [CH3COO]−, [CCl3COO]−, [CF3COO]−) were prepared and characterized by 1H NMR,13C NMR, FT-IR, TGA,UV-vis and elemental analysis. The more acidic ILs [DISM][CCl3COO] and [DSIM][CF3COO] were efficiently utilized as recyclable catalysts for the preparation of 14H-dibenzo[a,j]xanthene and 1,8-dioxo-decahydroacridine derivatives in short times under solvent-free conditions at 80–100 °C with excellent yields. The above two ILs could be effectively utilized as catalysts for the synthesis of 1,8-dioxo-decahydroacridine in water at the same temperature.
Introduction
The great capability of ionic liquids to replace volatile organic solvents offers a new and environmentally benign approach toward modern chemical processes.1 The implementation of task-specific ionic liquids with special functions further enhances the versatility of ionic liquids for those chemical reactions where both reagents and medium are coupled.2 By virtue of the incorporated functional groups, these unique salts can act not only act as solvents but also as catalysts in an array of synthetic, separation and electrochemical applications.3 Their solubility can also be tuned readily depending on the nature of the cations and anions so that they can phase separate from organic as well as aqueous media.4 The preparation and application of this dual nature of acidic ionic liquids in organic reactions overcomes the common problems of traditional molecular solvents and acid catalysts. These ILs are non-flammable, thermally stable and exhibit negligible vapour pressure and are recyclable. The higher potential of 1,3-disulfonic acid imidazolium ILs in organic synthesis has encouraged us to develop new members of the [DSIM][X] series where X = [CH3COO]−, [CCl3COO]−, [CF3COO]−.5,6 In addition, we have also tried to observe the efficiency of [DSIM][X] ionic liquids for the one pot synthesis of 1,8-dioxo-decahydroacridine 4 and dibenzoxanthene 5 derivatives in environmentally benign conditions. These heterocyclic compounds have wide applications in medicinal chemistry and material science.7,8 The traditional Brønsted or Lewis acid catalyzed synthesis of 1,8-dioxo-decahydroacridine derivatives has been performed in organic solvents such as HOAc,9a CH3CN9b and DMF.9c These conventional acids are often noxious, corrosive and non-recyclable, despite their high catalytic activity.9d The use of ionic liquids simplified the reaction conditions in terms of product selectivity, reaction time, recycling of the catalyst and isolation of the product.10 For the synthesis of dibenzoxanthene derivatives, a few reports have also utilized ionic liquids as catalyst or medium.11 This work concerns the synthesis, characterization and applications of 1,3-disulfonic acid imidazolium carboxylate ionic liquids and their applications in the synthesis of both classes of heterocyclic compounds.Results and discussion
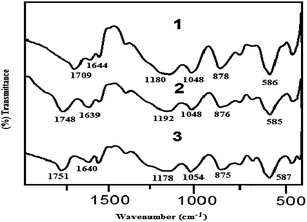
In continuation of our work12 on the development of environmentally benign routes for organic synthesis, we synthesised three new members of 1,3-disulfonic acid imidazolium carboxylate ionic liquids [DSIM][X] where X = [CH3COO]−, [CCl3COO]−, [CF3COO]− from the reactions of 1,3-disulfonic acid imidazolium chloride [DSIM][Cl] and three different carboxylic acids (AcOH, TCA and TFA) at room temperature stirring5a (Scheme 1).The FT-IR spectra (Fig. 1) of these ILs showed three strong absorption bands at 1178–1192, 1048–1054 and 586–587 cm−1 corresponding to S–O asymmetric and symmetric stretching and bending vibration of –SO3H groups. The peaks around 875–878 cm−1 expressed the N–S stretching vibration. The carboxylate anions gave strong asymmetric bands around 1709–1751 cm−1 in the same region of imidazolium ions. The –OH groups of the ionic liquids indicated broad and strong peaks at 3000–3660 cm−1. In 1H NMR spectra the characteristic acidic protons of two –SO3H groups of the ILs appeared in 14.2 and 11.3–13.4 ppm respectively. The presence of carbonyl signals at 158.6–172 ppm in 13C NMR spectra confirmed the existence of carboxylate anions in these ionic liquids. The structures were further supported by the elemental analysis data.The acidity of the ILs was determined on an UV-Visible spectrophotometer using 4-nitroaniline as basic indicator by following the Hammett equation that already reported in literature.13 The absorbance of the basic indicator [I] were determined in ionic liquid solutions which decreases with increasing the acidity of ionic liquids. The protonated form [HI]+ of the indicator never appeared because of low molar absorptivity. The Hammett function H° for each ionic liquids was calculated using eqn (1) by measuring the absorption differences [I]/[IH]+.
| H° = pK(I)aq. + log[I]/[IH]+ | (1) |
The thermo gravimetric analysis of the three ionic liquids showed (Fig. 3) slight weight loss below 100 °C due to absorbed moisture. All these ionic liquids were thermally stable in the temperature range of 260–281 °C.
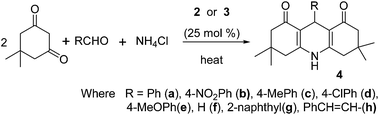
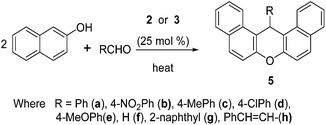
After calculating the acidity of ILs, we optimized the catalytic activity for the model synthesis of 1,8-dioxo-decahydroacridine 4a and dibenzoxanthene 5a in various conditions (Table 2). As a reaction medium the three ionic liquids didn't yield any product at ambient temperature for 4 hours. But at 100 °C the above reactions produced excellent yields within 10–15 minutes in [DSIM][CF3COO]and [DSIM][CCl3COO] ILs as reaction medium (Table 2, entries 2, 4, 10, 12) while [DSIM][CH3COO] ionic liquid showed less amount of product (Table 2, entries 1, 9). The best catalytic activity observed in solvent-free medium with 25 mol% of 3 at 90 °C for the synthesis of dibenzoxanthene(Table 2, entry 14) whereas it was 80 °C for the 1,8-dioxo-decahydroacridine(Table 2, entry 6). Using 25 mol% of ionic liquid 2, the product formation was decreased at 80 °C as compared to 100 °C under solvent-free condition (Table 2, entries 3, 7). Both ILs were effective for the formation of 4a in water at 90 °C with the optimized amount in short time (Table 2, entry 8).
| Entry | IL (mol%) | T (°C) | Time (min) | % of yielda |
|---|---|---|---|---|
| a Isolated yields.b Using 1 mL of water. | ||||
| For acridine derivatives 4a | ||||
| 1 | 1(100) | 100 | 25 | 65 |
| 2 | 2(100/50) | 100 | 10/10 | 98/98 |
| 3 | 2(25/10) | 100 | 10/45 | 96/65 |
| 4 | 3(100/50) | 100 | 10/10 | 100/100 |
| 5 | 3(25/10) | 100 | 10/45 | 100/82 |
| 6 | 3(25) | 90/80/70 | 10/10/25 | 100/100/70 |
| 7 | 2(25) | 80/90 | 20 | 70 |
| 8 | 2(25)/3(25) | 90 | 25 | 93b/98b |
| For dibenzoxanthene derivatives 5a | ||||
| 9 | 1(100) | 100 | 30 | 70 |
| 10 | 2(100/50) | 100 | 15/30 | 95/93 |
| 11 | 2(25/10) | 100 | 20/45 | 92/44 |
| 12 | 3(100/50) | 100 | 15/15 | 96/98 |
| 13 | 3(25/10) | 100 | 15/45 | 97/70 |
| 14 | 3(25) | 90/80/70 | 15/25/40 | 97/80/65 |
| 15 | 2(25) | 80/90 | 45 | 55 |
| 16 | 2(25)/3(25) | 100 | 1 h | 48b/60b |
Interestingly, the synthesis of 4a was possible only up to 48–60% yields in water at 100 °C along with unreacted 2-naphthol and aldehydes (Table 2, entry 16). It may be due to heterogeneous phases of 2-naphthol in water medium. The standardized conditions were extended with various types of aldehydes using ILs 2 and 3 as catalysts for the synthesis of other derivatives of these heterocyclic compounds. All the results were included in Tables 3 and 4. The results were observed to be satisfactory irrespective of the nature of aldehydes except with cinnamaldehyde and furaldehyde in both cases.
| Entry | R | Time (min) | % of yielda,b | Mp (°C) | ||
|---|---|---|---|---|---|---|
| Solvent free | In water | Solvent free(A/B) | In water(C/D) | |||
| a Method A: using 25 mol% of ionic liquid 2 at 100 °C; B: using 25 mol% of ionic liquid 3 at 80 °C; C: using 25 mol% of ionic liquids 2 at 90 °C; D: using 25 mol% of ionic liquid 3 at 90 °C.b All the products were characterized by 1NMR, 13CNMR, FT-IR and CHN analysis. | ||||||
| 1 | a | 10 | 25 | 96/100 4a | 93/98 4a | 287 (ref. 15) |
| 2 | b | 10 | 30 | 96/100 4b | 92/96 4b | 286 (ref. 16) |
| 3 | c | 15 | 35 | 94/98 4c | 90/94 4c | 279 (ref. 15) |
| 4 | d | 15 | 35 | 96/97 4d | 93/94 4d | 300 (ref. 15) |
| 5 | e | 15 | 35 | 95/99 4e | 90/96 4e | 280 (ref. 15) |
| 6 | f | 20 | 40 | 83/85 4f | 80/83 4f | 156 |
| 7 | g | 15 | 40 | 95/97 4g | 92/93 4g | 204 |
| 8 | h | 15 | 35 | 94/97 4h | 90/94 4h | 215 |
| Entry | R | Time (min) (A/B)a | % of yieldb | Mp (°C) |
|---|---|---|---|---|
| a Method A: using 25 mol% of ionic liquid 2 at 100 °C; B: using 25 mol% of ionic liquid 3 at 90 °C.b All the products were characterized by 1NMR, 13CNMR, FT-IR and CHN analysis. | ||||
| 1 | a | 20/15 | 92/97 5a | 186 (ref. 17) |
| 2 | b | 25/20 | 88/94 5b | 312 (ref. 17) |
| 3 | c | 20/15 | 90/93 5c | 228 (ref. 17) |
| 4 | d | 25/20 | 85/94 5d | 288 (ref. 17) |
| 5 | e | 20/15 | 92/97 5e | 204 (ref. 17) |
| 6 | f | 30/35 | 78/85 5f | 135 |
| 7 | g | 20/15 | 90/96 5g | 196–197 |
| 8 | h | 25/20 | 91/97 5h | 181 (ref. 18) |
The reusability of the ionic liquids 2 and 3 were expressed by using bar diagram (Fig. 4) for three consecutive runs for the synthesis of 1,8-dioxo-decahydroacridine 4a and dibenzoxanthene 5a under the optimized reaction conditions in solvent-free medium. Recyclability study indicated the high catalytic activity of the two ILs even after the third run with slight increasing reaction time.
Conclusions
In summary, a new group of 1,3 disulfonic acid imidazolium carboxylate ionic liquids was prepared and characterized by different analytical techniques. The acidity order of these ionic liquids in Hammett plot was identical with their catalytic activity for the one pot synthesis of 1,8-dioxodecahydroacridine and dibenzoxanthene derivatives in solvent-free medium in good to excellent yields.Experimental section
Experimental procedure
Acknowledgements
The authors are thankful to Sophisticated Analytical Instrumentation Centre, Tezpur University, for analyses of various samples for this work and also to CSIR, New Delhi, India for granting a research project no. 02(0067)/12/EMR-II to R.B.Notes and references
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3773–3789 CrossRef CAS; H. Olivier-Bourbigou, L. Magna and D. Morvan, Appl. Catal., A, 2010, 373, 1–56 CrossRef PubMed.
- (a) E. D. Bates, R. D. Mayton, I. Ntai and J. H. Davis Jr, J. Am. Chem. Soc., 2002, 124, 926–927 CrossRef CAS PubMed; (b) J. Fraga-Dubreuil and J. P. Bazureau, Tetrahedron Lett., 2001, 42, 6097–6100 CrossRef CAS.
- (a) S. Lee, Chem. Commun., 2006, 1049–1063 RSC; (b) M. Lombardo, F. Pasi and C. Trombini, Green Chem., 2007, 9, 321–322 RSC; (c) W. Chen, Y. Y. Zhang and L. B. Zhu, J. Am. Chem. Soc., 2007, 129, 13879–13886 CrossRef CAS PubMed.
- (a) W. S. Miao and T. H. Chan, Acc. Chem. Res., 2006, 39, 897–908 CrossRef CAS PubMed; (b) J. Vitz, D. H. Mac and S. Legoupy, Green Chem., 2007, 9, 431–433 RSC; (c) S. Doherty, P. Goodrich, C. Hardcare and C. Paun, Adv. Synth. Catal., 2008, 350, 295–302 CrossRef CAS PubMed.
- (a) F. Shirini and N. G. Khaligh, J. Mol. Liq., 2013, 177, 386–393 CrossRef CAS PubMed; (b) M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, A. Zare, H. G. Kruger, Z. Asgari, V. Khakyzadeh and M. Kazem-Rostami, J. Org. Chem., 2012, 77, 3640–3645 CrossRef CAS PubMed and references cited there in; (c) M. A. Zolfigol, V. Khakyzadeh, A. R. Moosavi-Zare, A. Zare, S. B. Azimi, Z. Asgari and A. Hasaninejad, C. R. Chim., 2012, 15, 719–736 CrossRef CAS PubMed.
- (a) M. A. Zolfigol, V. Khakyzadeha, A. R. Moosavi-Zarea, G. Chehardoli, F. Derakhshan-Panaha, A. Zare and O. Khalediana, Sci. Iran., Trans. C, 2012, 19, 1584–1590 CrossRef CAS PubMed; (b) M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare and A. Zare, Org. Prep. Proced. Int., 2010, 42, 95–102 CrossRef CAS; (c) A. Zare, T. Yousofi and A. R. Moosavi-Zare, RSC Adv., 2012, 2, 7988–7991 RSC; (d) A. Zare, F. Abi, A. R. Moosavi-Zare, M. H. Beyzavi and M. A. Zolfigol, J. Mol. Liq., 2013, 178, 113–121 CrossRef CAS PubMed; (e) M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, A. Zare, Z. Asgari, V. Khakyzadeha and A. Hasaninejad, J. Ind. Eng. Chem., 2013, 19, 721–726 CrossRef CAS PubMed.
- (a) Y. M. Shchekotikhin, T. G. Nikolaeva, G. M. Shub and A. P. Kriven'ko, Pharm. Chem. J., 2001, 35, 206–208 CrossRef CAS; (b) M. Kidwai and D. Bhatnagar, Tetrahedron Lett., 2010, 51, 2700–2703 CrossRef CAS PubMed; (c) S. A. Gamage, J. A. Spicer, G. J. Atwell, G. J. Finlay, B. C. Baguley and W. A. Denny, J. Med. Chem., 1999, 42, 2383–2393 CrossRef CAS PubMed.
- (a) A. Kumar, S. Sharma, R. A. Maurya and J. Sarkar, J. Comb. Chem., 2010, 12, 20–24 CrossRef CAS PubMed; (b) H. N. Hafez, M. I. Hegab, I. S. Ahmed-Farag and A. B. A. El-Gazzar, Bioorg. Med. Chem. Lett., 2008, 18, 4538–4543 CrossRef CAS PubMed; (c) V. B. Bojinov, I. P. Panova and D. B. Simeonov, Dyes Pigm., 2009, 83, 135 CrossRef CAS PubMed; (d) M. Sibrian-Vazquez and R. Strongin, Supramol. Chem., 2009, 21, 107–110 CrossRef CAS PubMed.
- (a) N. Martin, M. Quinteiro, C. Seoane, L. Soto, A. Mora, M. Suarez, E. Ockoa and A. Morals, J. Heterocycl. Chem., 1995, 32, 235–238 CrossRef CAS PubMed; (b) B. Das, P. Thirupathi, I. Mahender, V. S. Reddy and Y. K. Rao, J. Mol. Catal. A: Chem., 2006, 247, 233–239 CrossRef CAS PubMed; (c) V. A. Chebanov, V. E. Saraev, K. M. Kobzar, S. M. Desenko, V. D. Orlov and E. A. Gura, Chem. Heterocycl. Compd., 2004, 40, 475–479 CrossRef CAS; (d) R. J. Sarma and J. B. Baruah, Dyes Pigm., 2005, 64, 91–92 CrossRef CAS PubMed and references cited therein.
- (a) E. Rajanarendar, M. N. Reddy and F. P. Shaik, Indian J. Chem., 2011, 50 B, 245–252 Search PubMed; (b) D. Q. Shi, S. N. Ni, F. Yang, J. W. Shi, G. L. Dou, X. Y. Li and X. S. Wang, J. Heterocycl. Chem., 2008, 45, 653–660 CrossRef CAS PubMed.
- (a) K. Gong, D. Fang, H. L. Wang, X. L. Zhou and Z. L. Liu, Dyes Pigm., 2009, 80, 30–33 CrossRef CAS PubMed; (b) S. Kantevari, M. V. Chary, A. P. R. Das, S. V. N. Vuppalapati and N. Lingaiah, Catal. Commun., 2008, 9, 1575–1578 CrossRef CAS PubMed; (c) K. Y. Pratibha, V. Athindranath and S. M. S. Chauhan, Synth. Commun., 2008, 38, 637–648 CrossRef; (d) A. R. Hajipour, Y. Ghayeb, N. Sheikhan and A. Ruoho, Synlett, 2010, 5, 741–744 CrossRef PubMed; (e) P. Kumari, V. Yathindranath and S. M. Chauhan, Synth. Commun., 2008, 38, 637–648 CrossRef CAS.
- R. Borah, A. K. Dutta, P. Sarma, C. Dutta and B. Sarma, RSC Adv., 2014, 4, 10912–10917 RSC.
- (a) W. Yuan-Yuan, L. Wei and D. Li-Yi, Chin. J. Chem., 2008, 26, 1390–1394 CrossRef PubMed; (b) C. Thomazeau, H. Olivier-Bourbigou, L. Magna, S. Luts and B. Gilbert, J. Am. Chem. Soc., 2003, 125, 5264–5265 CrossRef CAS PubMed.
- A. Albert and E. P. Serjeant, The Determination of Ionization Constants: A Laboratory Manual, Chapman and Hall, New York, 3rd edn, 1984 Search PubMed.
- S. M. Vahdat and M. Akbari, Orient. J. Chem., 2011, 27, 1573–1580 CAS.
- B. Saeed, C. Fatemeh, D. Fatemeh and B. H. Reza, Chin. J. Chem., 2009, 27, 1953–1956 CrossRef PubMed.
- B. Rajitha, B. S. Kumar, Y. T. Reddy, P. N. Reddy and N. Sreenivasulu, Tetrahedron Lett., 2005, 46, 8691–8693 CrossRef CAS PubMed.
- R. Kumar, G. C. Nandi, R. K. Verma and M. S. Singh, Tetrahedron Lett., 2010, 51, 442–445 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: This documents contains the 1HNMR and 13C NMR spectral data of ILs (1, 2 and 3), 1,8-dioxo-decahydroacrdine (4) and dibenzoxanthene (5)with the soft copies of spectra. See DOI: 10.1039/c4ra07323a |
| This journal is © The Royal Society of Chemistry 2014 |