Advanced amorphous nanoporous stannous oxide composite with carbon nanotubes as anode materials for lithium-ion batteries
Wenjuan Jianga,
Weiyao Zenga,
Zengsheng Ma*a,
Yong Pana,
Jianguo Lin*a and
Chunsheng Lub
aKey Laboratory of Low Dimensional Materials and Application Technology of Ministry of Education, School of Materials Science and Engineering, Xiangtan University, Xiangtan 411105, Hunan, China. E-mail: zsma@xtu.edu.cn; lin_j_g@xtu.edu.cn; Fax: +86-731-58293577; Tel: +86-731-58293577
bDepartment of Mechanical Engineering, Curtin University, Perth, WA 6845, Australia
First published on 12th August 2014
Abstract
An amorphous nanoporous stannous oxide (ANSO) composite with carbon nanotubes (CNTs) electrode material is synthesized by electrodeposition and anodic oxidation methods. The ANSO@CNTs as an anode material for lithium-ion batteries exhibits a highly reversible specific capacity, stable cycling ability and good rate capabilities. The first discharge capacity is as high as 1895.3 mA h g−1 with a reversible capacity of 1608.2 mA h g−1 at a current density of 100 mA g−1. Furthermore, an electrochemical performance with a highly reversible capacity and enhanced rate capability can be found due to the highly uniform distribution and superior electrochemical and mechanical performances of the CNTs. The preparation approach for the ANSO@CNTs anode material reported in this paper may be applied to large-scale production of anode materials for high performance lithium-ion batteries.
1. Introduction
With the demand for higher energy density, higher power density, good safety performance, long life cycles, and low pollution, lithium-ion batteries are used as an ideal power storage device.1–3 The anode material has a significant impact on the specific capacity and cyclability of lithium-ion batteries. Graphite is commonly used as the anode material with a theoretical specific capacity of 372 mA h g−1. So far, the specific capacity of the carbon anode material has been close to the theoretical specific capacity, so it is unlikely to further improve the lithium storage properties.Tin oxide (TO) is a very promising high performance anode material.4 It has a theoretical specific capacity of 990 mA h g−1, which is almost three times that of carbon materials, attracting the extensive attention of scientists from all over the world. Idota et al. have reported that amorphous stannous oxide possesses a low intercalation potential, stable cycle ability, and the reversible specific capacity can reach 600 mA h g−1.5 Although the first discharge capacity of these anode materials is high, TO suffers from severe capacity fading during cycling because of the large volume deformation that occurs during the charge–discharge process.6,7
There are a large number of pores in mesoporous materials, which can probably relieve the huge volume deformation during the charge–discharge processes. Shiva et al. prepared porous SnO2 by the hydrothermal method with a mesoporous diameter of about 2–7.5 nm.8 Using porous SnO2 as the anode materials for a lithium ion battery, the cyclability was much better than that of the pure Sn anode materials, and the coulomb efficiency of first cycle can reach 95%. Uchiyama et al. also obtained a single crystal nano-material of reticular SnO2 by the hydrothermal method, and the reversible specific capacity of the first cycle was about 900 mA h g−1.9 Although these studies have prepared TO anode materials with high specific capacity, the processes were complex and expensive. Hence, there is a need to improve the process of TO anode materials for industrial production.
Anodic oxidation can grow an oxide film on the anode surface using a metal or metal alloy as the anode in a specific electrolyte. Yang et al. have used ammonium fluoride solution as the electrolyte to successfully prepare titania nanotube arrays by the anodic oxidation method.10 Courtney and Dahn found that the voltage platform of 1.58 V of TO anode materials at the first cycle indicates the Li2O growth at the anode because of difference in the Gibbs free energy (Li2O: 562.1 kJ mol−1; SnO2: 256.8 kJ mol−1).11 Li2O growth is an irreversible process, resulting in a high irreversible capacity in the first cycle. Moreover, due to their relatively low melting points, tin atoms are easily gathered as clusters, and then a two-phase region appears in TO anode materials, leading to the capacity fading during the cycling. Because of the above-mentioned problems, scientists have improved TO anode materials by impurity doping.12–14 Due to the good electrochemical and mechanical performances, CNTs have also received wide attention in the field of active materials for lithium-ion batteries.15–18
In this article, an ANSO@CNTs anode material is prepared with composite electrodeposition and anodic oxidation, as shown in Fig. 1. Good electronic conductivity and mechanical properties are created by introducing the CNTs because its porous structure facilitates liquid electrolyte diffusion into the active materials. It is shown that the ANSO@CNTs anode material possesses perfect cycling performance and good rate capability.
2. Experimental
2.1. Preparation of CNTs@tin as precursors for anodization
For the electrochemical deposition of CNTs@tin, CNTs should be stirred with PVP (weight ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 12 hours, and then the mixture is added into the tin plating solution. A copper sheet was used as the working electrode. It was prepared by soaking in a degreaser containing caustic soda, natronite, sodium phosphate, and sodium silicate for 5 minutes and then diluted sulfuric acid to remove oil contamination and the native surface oxide, respectively, and then it was cleaned with distilled water. An ordinary tin block was adopted as the counter electrode. A constant cathodic current of 1 A dm−2 was applied for 5 minutes. The plating tank was under ultrasonic bath conditions with an ultrasonic frequency of about 45 KHz.
1) for 12 hours, and then the mixture is added into the tin plating solution. A copper sheet was used as the working electrode. It was prepared by soaking in a degreaser containing caustic soda, natronite, sodium phosphate, and sodium silicate for 5 minutes and then diluted sulfuric acid to remove oil contamination and the native surface oxide, respectively, and then it was cleaned with distilled water. An ordinary tin block was adopted as the counter electrode. A constant cathodic current of 1 A dm−2 was applied for 5 minutes. The plating tank was under ultrasonic bath conditions with an ultrasonic frequency of about 45 KHz.
2.2. Anodization of CNTs@tin
For the preparation of the ANSO@CNTs anode material, CNTs@tin films in Section 2.1 and graphite flakes were used as the working electrode (anode) and the counter electrode (cathode), respectively. The distance between the anode and cathode was kept at 5 cm. A constant potential of 8 V was applied to the anode in an electrolyte consisting of 0.1 M oxalic acid at room temperature. An EG&G 263A potentiostat/galvanostat was used in the electrochemical depositions.2.3. Electrochemical measurements
The electrochemical experiments were carried out using coin cells. The as-prepared samples were cut into wafers used as the working electrode. The celgard 2325 microporous membrane was used as the separator. The electrolyte was 1 mol L−1 LiPF6 dissolved in a mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The lithium sheet was used as both the counter and reference electrodes. CR2025-type coin cells were assembled in an argon-filled glove box and galvanostatically discharged and charged in a voltage range from 0.01–2.5 V by a battery testing system. Cyclic voltammetry (CV) test has been carried out on an electrochemical workstation (Zahner IM6ex) over the potential range of 0.01–2.5 V versus Li/Li+ at a scanning rate of 0.1 mV s−1. Electrochemical impedance spectra of the electrodes were recorded from 1000 KHz to 0.1 mHz with the amplitude of 5 mV.
1). The lithium sheet was used as both the counter and reference electrodes. CR2025-type coin cells were assembled in an argon-filled glove box and galvanostatically discharged and charged in a voltage range from 0.01–2.5 V by a battery testing system. Cyclic voltammetry (CV) test has been carried out on an electrochemical workstation (Zahner IM6ex) over the potential range of 0.01–2.5 V versus Li/Li+ at a scanning rate of 0.1 mV s−1. Electrochemical impedance spectra of the electrodes were recorded from 1000 KHz to 0.1 mHz with the amplitude of 5 mV.
3. Results and discussion
3.1. Microstructural characterization
SEM images of the ANSO@CNTs anode material by the one-step anodization in 0.1 M oxalic acid at a potential of 8 V are shown in Fig. 2(a) and (b). As can be seen, nanopores are distributed randomly on the surface with a diameter ranging from about 1 nm to over 20 nm. A dense array of parallel nanochannels, almost perpendicular to the substrate, can be observed clearly. The nanopore structure of tin oxide is similar to what has been observed in previous investigations.19,20 The microstructures and morphologies of the prepared samples are characterized by X-ray diffraction (XRD, Bruker D8 Advance), transmission electron microscopy (TEM, FEI, Tecnai G2 F30 S-Twin), and scanning electron microscopy (Field-Emission SEM, S-4800, Hitachi, Japan). N2 adsorption–desorption isotherms were measured using a Micromeritics ASAP 2010 (USA) analyzer at the liquid nitrogen boiling temperature. CNTs with a diameter of 10 nm were uniformly distributed in the nanoporous SnO layer, as shown in Fig. 2(c). | ||
| Fig. 2 SEM images of the ANSO@CNTs anode material: (a) an overview, (b) a magnified image and (c) TEM image. | ||
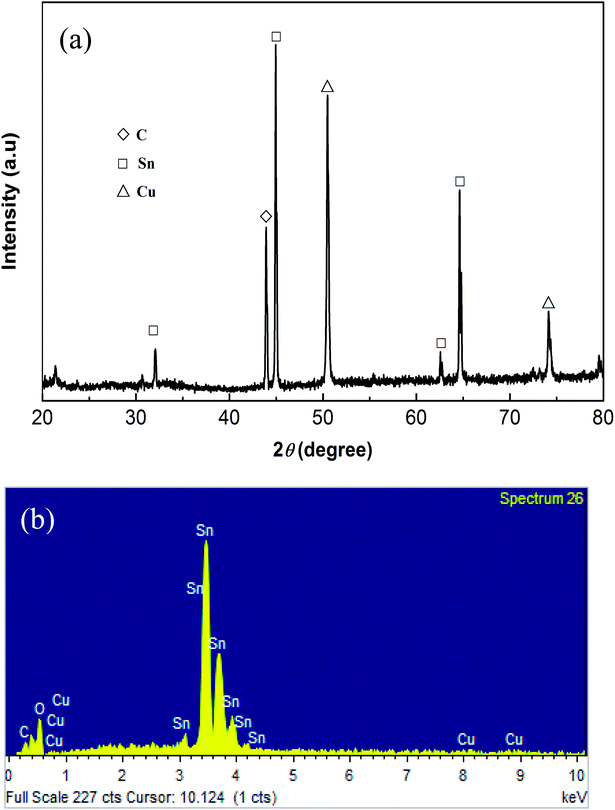
Fig. 3(a) is the XRD spectrum showing peaks for metallic cooper, metallic tin and traces of CNTs. The SnO of the as-prepared ANSO@CNTs anode material was found to be amorphous and the dark color of the oxide film suggested formation of SnO during anodization (Fig. 2(c)). Moreover, the ratio of the Sn atom and O atom was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, corresponding to SnO, and the content of CNTs is 7.57 wt%, as seen from the EDS map in Fig. 3(b).
1, corresponding to SnO, and the content of CNTs is 7.57 wt%, as seen from the EDS map in Fig. 3(b).
N2 adsorption–desorption isotherms and pore size distribution of the final ANSO@CNTs anode material are shown in Fig. 4. The nitrogen adsorption–desorption isotherms present obvious pressure hysteresis, indicating that the ANSO@CNTs anode material is porous.21,22 The Brunauer–Emmett–Teller (BET) surface area of ANSO@CNTs is 53.4 m2 g−1, and the total pore volume is 0.151 cm3 g−1. It should be noted that some nanopores smaller than 2 nm exist in the ANSO@CNTs anode material, implying a great benefit to the reversible specific capacity because lithium ions can be stored in these nanopores, which further enhances the high rate capability.23,24
 | ||
| Fig. 4 (a) Nitrogen adsorption–desorption isotherms of the ANSO@CNTs anode material, (b) porosity distribution by the Original Density Functional Theory Model. | ||
3.2. Electrochemical properties of the ANSO@CNTs anode material
The discharge–charge cycles were tested at a specific current of 100 mA g−1 by using coin cells, and the 1st, 2nd and 10th discharge–charge curves are shown in Fig. 5(a). The 1st discharge and charge specific capacities of the ANSO@CNTs anode material are as high as 1895 and 1608 mA h g−1, respectively. The initial coulombic efficiency can reach 85% calculated based on the first discharge–charge specific capacities. The irreversible capacity loss, ∼15%, is mainly due to electrolyte decomposition forming a thick solid-electrolyte interface layer on the electrode surface.25–27The electrochemical behavior of the ANSO@CNTs anode material using cyclic voltammetry (CV) analysis at a scanning rate of 0.1 mV s−1 is shown in Fig. 5(b). In the reduction process, the hump above 0.5–0.75 V could be attributed to an irreversible separation of SnO to Sn and Li2O.28 The peak around 0.3 V reflects the formation of a solid-electrolyte interface (SEI) leading to a large irreversible capacity fade that corresponds with the low columbic efficiency in the first cycle. Meanwhile, in the oxidation process, three peaks were recorded at about 0.6, 0.7 and 0.8 V. The peak at 0.6 V corresponds to lithium extraction from the CNTs,26 and the peak at 0.7 V represents the de-alloying process of Li+ ions, while the following weak peak at 0.8 V could be ascribed to the reaction of Sn and Li2O.29,30
Fig. 5(c) shows the cycle performance and coulombic efficiency during cycling of the ANSO@CNTs anode material. The coulombic efficiency remains near 97%. It can be observed that the cycling stability in the first 22 cycles is low. From the 2nd cycle, the discharge capacity decreases from 1429.3 mA h g−1 to 839.4 mA h g−1 after the following 22 cycles and then gradually increases to 890 mA h g−1 after 50 cycles. The increasing specific capacity may be due to the formation of an activation process of the ANSO@CNTs. Even at higher current densities of 200, 300, 500, and 1000 mA g−1, the ANSO@CNTs still maintains a perfect cycling stability. After 50 cycles, the discharge capacity is as high as 732.5 mA h g−1 at the current density of 100 mA g−1, as shown in Fig. 5(d). However, the bare ANSO without CNTs did not fare as well, retaining a charge capacity of 513 mA h g−1 after 50 cycles compared to 890 mA h g−1 of ANSO@CNTs at the current density of 100 mA g−1. The ANSO also shows a poor cycling performance in Fig. 5(c). Therefore, the ANSO@CNTs shows much better charge capacity and cycling performance compared with the bare ANSO, which is attributed to the good electrochemical and mechanical performances of CNTs in SnO nanoporous layer.
Fig. 6 shows electrochemical impedance spectroscopy of ANSO@CNTs. It can be seen that the ANSO@CNTs anode materials (∼59 Ω) has a much lower charge transfer resistance than that of bare ANSO without CNTs (170 Ω), indicating a faster Li+ diffusion and electron transfer in ANSO@CNTs.
 | ||
| Fig. 6 Electrochemical impedance spectra of (a) ANSO@CNTs anode material and (b) ANSO anode material. | ||
4. Conclusions
In summary, ANSO@CNTs was prepared through composite electrodeposition and anodic oxidation. The ANSO@CNTs anode materials exhibited a superior first discharge capacity of 1895 mA h g−1 with a reversible capacity of 1608 mA h g−1 at a current density of 100 mA g−1. After 40 cycles at different current densities of 100, 200, 500 and 1000 mA g−1, the charge capacity still maintained at 732.5 mA h g−1 at a current density of 100 mA g−1. Compared with bare ANSO, the ANSO@CNTs exhibits enhanced Li-ion battery performance with a large reversible capacity and good cycling performance. The good electrochemical performance can be attributed to the following reasons: (1) the nanoporous material has a high reactivity and short path lengths for electron and Li+ transport; (2) large numbers of mesopores can provide huge buffer space for volume deformation during the charge–discharge process, improving the cycle performance; (3) uniformly inserted CNTs alleviated chalking of electrode during discharge–charge progress and improved the Li+ transport speed. The ANSO@CNTs can be a promising alternative anode material used for high-capacity lithium-ion batteries.Acknowledgements
We gratefully acknowledge financial support from the National Natural Science Foundation of China (no. 11372267 and 11102176), the National High Technology Research and Development Program of China (863 Program) (2013AA032502), and the Emerging Strategic Industries of Hunan Province (2012GK4075).References
- J. Cheng, Y. Pan, J. Zhu, Z. Li, J. Pan and Z. Ma, J. Power Sources, 2014, 257, 192–197 CrossRef CAS PubMed.
- Y. Wang, Z. Feng, C. Zhang, L. Yu, C. Shi, J. Chen, J. Hu and X. Liu, Nanoscale, 2013, 5, 3704–3712 RSC.
- Y. Wang, Z. Feng, J. Chen, C. Zhang, X. Liu and J. Hu, Solid State Commun., 2012, 152, 1577–1580 CrossRef CAS PubMed.
- X. Zhou, Z. Dai, S. Liu, J. Bao and Y. G. Guo, Adv. Mater., 2014, 26, 3943–3949 CrossRef CAS PubMed.
- Y. Idota, T. Kubota, A. Matsufuji, Y. Maekawa and T. Miyasaka, Science, 1997, 276, 1395–1397 CrossRef CAS.
- M. O. Guler, O. Cevher, T. Cetinkaya, U. Tocoglu and H. Akbulut, Int. J. Energy Res., 2014, 38, 487–498 CrossRef CAS PubMed.
- Z. Ma, T. Li, Y. Huang, J. Liu, Y. Zhou and D. Xue, RSC Adv., 2013, 3, 7398–7402 RSC.
- K. Shiva, M. S. R. N. Kiran, U. Ramamurty, S. Asokan and A. Bhattacharyya, J. Solid State Electrochem., 2012, 16, 3643–3649 CrossRef CAS PubMed.
- H. Uchiyama, S. Nakanishi and H. Kozuka, J. Solid State Chem., 2014, 217, 87–91 CrossRef CAS PubMed.
- D. J. Yang, H. Park, H. G. Kim, S. J. Cho and W. Y. Choi, J. Electroceram., 2009, 23, 159–163 CrossRef CAS.
- I. A. Courtney and J. Dahn, J. Electrochem. Soc., 1997, 144, 2943–2948 CrossRef CAS PubMed.
- J. S. Chen, L. A. Archer and X. W. D. Lou, J. Mater. Chem., 2011, 21, 9912–9924 RSC.
- J. S. Chen and X. W. D. Lou, Small, 2013, 9, 1877–1893 CrossRef CAS PubMed.
- S. H. Lee, S. H. Jee, K. S. Lee, S. C. Nam and Y. S. Yoon, Electrochim. Acta, 2013, 87, 905–911 CrossRef CAS PubMed.
- M. Reddy, G. Subba Rao and B. Chowdari, Chem. Rev., 2013, 113, 5364–5457 CrossRef CAS PubMed.
- H. Song, N. Li, H. Cui and C. Wang, Electrochim. Acta, 2014, 120, 46–51 CrossRef CAS PubMed.
- L. Noerochim, J.-Z. Wang, S.-L. Chou, H.-J. Li and H.-K. Liu, Electrochim. Acta, 2010, 56, 314–320 CrossRef CAS PubMed.
- W. Lei, Y. Pan, Y. Zhou, W. Zhou, M. Peng and Z. Ma, RSC Adv., 2014, 4, 3233–3237 RSC.
- J. W. Lee, S. J. Park, W. S. Choi and H. C. Shin, Electrochim. Acta, 2011, 56, 5919–5925 CrossRef CAS PubMed.
- L. Zaraska, N. Czopik, M. Bobruk, G. D. Sulka, J. Mech and M. Jaskuła, Electrochim. Acta, 2013, 104, 549–557 CrossRef CAS PubMed.
- S. M. Paek, E. Yoo and I. Honma, Nano Lett., 2008, 9, 72–75 CrossRef PubMed.
- L. Yu, C. Falco, J. Weber, R. J. White, J. Y. Howe and M. M. Titirici, Langmuir, 2012, 28, 12373–12383 CrossRef CAS PubMed.
- Y. G. Guo, J. S. Hu and L. J. Wan, Adv. Mater., 2008, 20, 2878–2887 CrossRef CAS PubMed.
- J. Zhu, D. Wang, L. Wang, X. Lang and W. You, Electrochim. Acta, 2013, 91, 323–329 CrossRef CAS PubMed.
- L. Ji, Z. Lin, M. Alcoutlabi and X. Zhang, Energy Environ. Sci., 2011, 4, 2682–2699 CAS.
- F. M. Hassan, Z. Chen, A. Yu, Z. Chen and X. Xiao, Electrochim. Acta, 2013, 87, 844–852 CrossRef CAS PubMed.
- P. Abellan, B. L. Mehdi, L. R. Parent, M. Gu, C. Park, W. Xu, Y. Zhang, I. Arslan, J. Zhang and C. Wang, Nano Lett., 2014, 14, 1293–1299 CrossRef CAS PubMed.
- G. A. Elia, S. Panero, A. Savoini, B. Scrosati and J. Hassoun, Electrochim. Acta, 2013, 90, 690–694 CrossRef CAS PubMed.
- J. Yao, X. Shen, B. Wang, H. Liu and G. Wang, Electrochem. Commun., 2009, 11, 1849–1852 CrossRef CAS PubMed.
- P. Lian, X. Zhu, S. Liang, Z. Li, W. Yang and H. Wang, Electrochim. Acta, 2011, 56, 4532–4539 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |