Anticancer (in vitro) and antimicrobial effect of gold nanoparticles synthesized using Abelmoschus esculentus (L.) pulp extract via a green route
Abstract
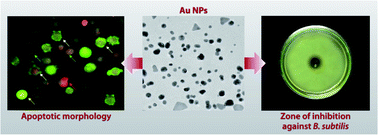
Green synthesis of gold nanoparticles (Au NPs) using Abelmoschus esculentus (L.) pulp extract has been elaborately studied and reported here. The Au NPs have been characterized using several techniques. Optical analysis indicates adequate stability of the synthesized Au NPs, while FTIR analyses the fact that phytochemicals present in the Abelmoschus esculentus (L.) pulp extract play the key role in stabilizing the Au NPs. Morphological study shows that the nanoparticles are mostly spherical in shape with an average particle size of ∼14 nm, and these results are comparable with the particle size obtained from XRD. The selected area electron diffraction pattern indicates the crystalline nature of the Au NPs, which is further confirmed from XRD studies. The present study also demonstrates the in vitro efficacy of Au NPs against Jurkat cells. Results show that the IC50 dose of Au NPs is capable of significantly elevating intracellular reactive oxygen species and diminishing mitochondrial membrane potential, indicating the effective involvement of apoptosis in cell death. Furthermore, the synthesized Au NPs show a sufficient degree of antimicrobial activity against different types of bacteria. These results clearly show that the Abelmoschus esculentus (L.) pulp synthesized Au NPs have excellent medicinal applications.

 Please wait while we load your content...
Please wait while we load your content...