Surface modification with thermoresponsive polymer brushes for a switchable electrochemical sensor†
Clément Comminges‡
*a,
Stefano Frascaa,
Martin Sütterlinb,
Erik Wischerhoffc,
André Laschewskyb and
Ulla Wollenberger*ab
aInstitut für Biochemie und Biologie, Universität Potsdam, Karl-Liebknecht-Str. 24-25, Haus 25, 14476 Potsdam-Golm, Germany. E-mail: uwollen@uni-potsdam.de; Fax: +49 331 977 5128; Tel: +49 331 977 5122
bInstitut für Chemie, Universität Potsdam, Karl-Liebknechtstraße 24-25, 14476 Potsdam-Golm, Germany
cFraunhofer-Institut für Angewandte Polymerforschung (IAP), Geiselbergstraße 69, 14476 Potsdam-Golm, Germany
First published on 5th September 2014
Abstract
Elaboration of switchable surfaces represents an interesting way for the development of a new generation of electrochemical sensors. In this paper, a method for growing thermoresponsive polymer brushes from a gold surface pre-modified with polyethyleneimine (PEI), subsequent layer-by-layer polyelectrolyte assembly and adsorption of a charged macroinitiator is described. We propose an easy method for monitoring the coil-to-globule phase transition of the polymer brush using an electrochemical quartz crystal microbalance with dissipation (E-QCM-D). The surface of these polymer modified electrodes shows reversible switching from the swollen to the collapsed state with temperature. As demonstrated from E-QCM-D measurements using an original signal processing method, the switch is operating in three reversible steps related to different interfacial viscosities. Moreover, it is shown that the one electron oxidation of ferrocene carboxylic acid is dramatically affected by the change from the swollen to the collapsed state of the polymer brush, showing a spectacular 86% decrease of the charge transfer resistance between the two states.
Introduction
Stimuli responsive polymers are macromolecules that can undergo structural changes as a response to external stimuli. The most common triggers for this transition are changes in temperature, pH or ionic strength.1 When such polymers are grafted on a substrate as a dense brush, interfacial properties such as surface adhesion, wettability or interfacial viscoelastic properties can be reversibly modified as result of transition induced structural changes.2–4 This makes such responsive brushes attractive candidates as switchable elements in electrochemical systems.5,6In the case of thermoresponsive polymers in aqueous media, the coil-to-globule transition is mostly characterized by a lower critical solution temperature (LCST). Below the transition temperature, the grafted polymer chains are strongly swollen and stretch away from the surface, whereas above the transition, such brushes expel most of the solvent, deswell and finally collapse on the substrate. The transition temperature is characteristic for the polymer studied and the specific aqueous solvent used. It can be conveniently tuned by co-polymerization of different monomers. Macromolecules constructed with short oligo(ethylene glycol) methacrylates (OEGMA) constitute an interesting class of thermoresponsive polymers for preparing biorelevant and biocompatible switchable surfaces.7 While the phase transition of these copolymers is reversible, the precise transition temperature of a given copolymer depends to some extent on system parameters such as molar mass and polydispersity, polymer concentration, and the ionic strength of the medium.7–11 Random copolymers of 2-(2-methoxyethoxy)ethyl methacrylate (MEO2MA) and OEGMA typically exhibit a LCST in water, which can be precisely adjusted by varying the co-monomer ratio.7,10,12–15 Interestingly, this LCST-type transition can be detected by electrochemical methods.16
We herein report a molecular architecture of a LCST polymer modified gold surface, and its interfacial properties in terms of charge transfer towards an electrochemical probe. The modular character of the straightforward coating process enables a priori the simple and versatile functionalization of such polymer modified electrodes. We also introduce differential E-QCM-D measurement to characterize the ability of the system to actively respond upon temperature variations in order to develop a switchable readout system exploiting the structural changes of the surface.
Experimental methods
Chemicals
Poly(4-styrenesulfonic acid) (PSS, Mw = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), Ferrocene carboxylic acid (FcCOOH), tris(hydroxymethyl)-aminomethane (tris), hydroxyethyl methacrylate (HEMA), Dithiobis(succinimidyl propionate) (DTSP), branched polyethyleneimine (PEI, Mw = 25
000 g mol−1), Ferrocene carboxylic acid (FcCOOH), tris(hydroxymethyl)-aminomethane (tris), hydroxyethyl methacrylate (HEMA), Dithiobis(succinimidyl propionate) (DTSP), branched polyethyleneimine (PEI, Mw = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), and azo-bis-isobutyronitrile (V-601) were purchased from Sigma (Steinheim, Germany) and used as received, when not specified otherwise. Poly(diallyldimethylammonium chloride) (PDADMAC, Mn = 230
000 g mol−1), and azo-bis-isobutyronitrile (V-601) were purchased from Sigma (Steinheim, Germany) and used as received, when not specified otherwise. Poly(diallyldimethylammonium chloride) (PDADMAC, Mn = 230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Mw = 570
000 g mol−1, Mw = 570![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) was prepared according to a published procedure.17
000 g mol−1) was prepared according to a published procedure.17
Surface modification: DTSP self assembled monolayer (SAM), coupling with PEI and layer by layer assembly
All surface modifications were monitored with an Electrochemical Quartz Crystal Microbalance with Dissipation (E-QCM-D), as it is a well suited method for monitoring the consecutive surface modification steps,18 using a Q-Sense E4-instrument (Q-Sense, AB, Göteborg, Sweden) incorporating a Q-Sense Electrochemistry Module, QEM 401 and polished AT-cut piezoelectric quartz crystals with gold electrodes with a fundamental frequency of 5 MHz as substrates. The gold substrates (disk for E-QCM-D and wires for electrochemical measurements) were cleaned according to a previously published procedure.19 Briefly, crystals were immersed in a mixture of ammonia (25%), hydrogen peroxide (30%), and Milli-Q water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), held at 70 °C for 10 min, rinsed extensively with Milli-Q water, and dried under a stream of nitrogen.
5), held at 70 °C for 10 min, rinsed extensively with Milli-Q water, and dried under a stream of nitrogen.
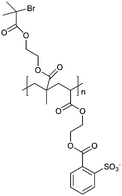
A first modifying layer consisting of a SAM of the functional disulfide DTSP was prepared by incubating the clean gold electrode in a 10 mmol l−1 DTSP solution in CHCl3 at 4 °C overnight. DTSP modified electrodes were rinsed with CHCl3 and water and assembled in the E-QCM-D module. PEI aqueous solution (1 g l−1) was flown (10 μl min−1) over the SAM to covalently couple the primary amine moieties of PEI to DTSP. Then, layer-by-layer assembly (LBL)20,21 of 4 alternate layer pairs of PSS and PDADMAC (0.2 g l−1 solution in 5 mmol l−1 Tris buffer pH 7.2) was applied in order to build a polyelectrolyte multilayer. Each deposition step was followed by rinsing with 5 mmol l−1 Tris buffer pH 7.2 to remove the loosely adhering molecules. The flow rate was always 50 μl min−1, if not otherwise mentioned, and the injected solution was changed only after a stable frequency signal was reached. Finally, the sulfonated polyanion macroinitiator MI (Scheme 1) (0.2 mg ml−1 in water), synthesized according to a published procedure,22 was adsorbed onto the terminal PDADMAC layer.
Surface initiated polymerization
The MI on top of the polyelectrolyte multilayer was used as starting point for the surface-initiated atom transfer radical random copolymerization (ATRP) of MEO2MA and OEGMA monomers (ratio 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5),23–25 which was catalysed with a copper 2,2′-bipyridine bromide complex (CuBr2bpy2). A stock solution of co-monomers and copper complex is bubbled with nitrogen and pumped into the E-QCM-D chamber. The polymerization was monitored by following both frequency shift and dissipation with time. The resulting modified surface will be referred to as the Polymer Modified Electrode (PME) in the following. The phase transition temperatures of the grafted polymer brushes PME were determined by E-QCM-D measurements using a temperature ramp of 0.2 ° min−1 from 15 °C to 38 °C in a 25 mmol l−1 TRIS buffer pH 8.
5),23–25 which was catalysed with a copper 2,2′-bipyridine bromide complex (CuBr2bpy2). A stock solution of co-monomers and copper complex is bubbled with nitrogen and pumped into the E-QCM-D chamber. The polymerization was monitored by following both frequency shift and dissipation with time. The resulting modified surface will be referred to as the Polymer Modified Electrode (PME) in the following. The phase transition temperatures of the grafted polymer brushes PME were determined by E-QCM-D measurements using a temperature ramp of 0.2 ° min−1 from 15 °C to 38 °C in a 25 mmol l−1 TRIS buffer pH 8.
Electrochemical measurements
Cyclic voltammetry and electrochemical impedance spectroscopy (EIS) were performed on a CH Instrument Model 750 A (Austin, USA) to characterize the electronic transfer for FcCOOH oxidation (5 mmol l−1 solution in citrate-phosphate buffer 0.1 mol l−1 pH 7.1). A thermostated 3 electrode cell comprising the polymer modified electrode (PME gold wire) as working electrode, Ag/AgCl, KCl 1 M reference and platinum wire as counter electrode were used. All potentials are referred to Ag/AgCl, KCl 1 M reference. DC potential for EIS measurements was chosen as the potential obtained at Iap/2 during cyclic voltammetry at 10 °C and 50 mV s−1 (310 mV for PME and 282 mV for the bare gold electrode). Amplitude and frequency range are 10 mV and 96 kHz to 374 mHz respectively. Charge transfer resistance (RCT) for the one electron oxidation of FcCOOH was determined by fitting from Re−(Rct/CPE) equivalent circuit using ZView software.Results and discussion
Surface modification
The E-QCM-D measurements show that PEI covalent grafting onto the DTSP modified gold substrate is completed after 1 h, with a 23 Hz negative frequency shift (ESI Fig. 1†) (3rd overtone), which corresponds to a mass of 136 ng cm−2 using the Sauerbrey equation (eqn (1)).
 | (1) |
The LBL assembly was monitored by E-QCM-D (Fig. 1a). A negative frequency shift is observed after each layer deposition. A final frequency shift of 350 Hz is measured for a chip covered by 4 bilayers of PSS/PDADMAC and one layer of macroinitiator. The corresponding dissipation is rather high (84 × 10−6) (ESI Fig. 2†) due to the flexibility of the polyelectrolyte chains, so the Sauerbrey equation was not applicable.27 Fig. 1b shows the on-line monitoring of the polymerization with E-QCM-D, which leads to 2700 Hz frequency decrease after 4.5 h of polymerization, indicating a large mass increase over the surface and 700 × 10−6 dissipation increase which is typical for the formation of a viscoelastic film.28 The proposed final PME architecture is represented on Fig. 1c. According to previous studies on similar structures,23 thicknesses of LBL under layer (∼40 nm), MI layer (∼20 nm) and polymer layer (∼70 nm) were obtained.
Phase transition of the responsive polymer
Fig. 2a and b show the resonance frequency and dissipation variation upon temperature changes. A bare gold chip and an assembled system without polymer layer show linear evolution of frequency and dissipation change with temperature, which are expected for solvent density and viscosity variation. However, frequency and dissipation change displays a totally different behavior for the PME. Upon temperature increase, a large negative frequency shift is observed. This is related to the collapsing of the polymer, passing from a stretched and viscoelastic form to a more rigid structure. According to Laloyaux et al.,29 this can be described as a two-step collapsing. Zone 1 corresponds to the fully swollen state, where frequency and dissipation data are close to the under layer behavior. In zone 2, dissipation is increasing whereas frequency decreases. This difference with the under layer response shows the beginning of the bulk brushes collapsing. The increase of dissipation in that zone indicates a metastable behavior of the brushes which appear to be more flexible. In zone 3, the surface brushes are fully collapsed, indicated by a large dissipation and frequency decrease. The frequency shows a minimum, indicating a maximal synergy between surface and bulk collapse. After this minimum, the frequency change is increasing again, indicating that surface collapse is progressively completed and the polymer chains are confined and almost no more flexible. In zone 4 and 4′, the film is behaving like the under layer as a rigid coating on the surface. Zones 3′ and 2′ are the reversible processes of surface and bulk re-swelling upon cooling.As the Au/under layer assembly behaves as a thick rigid film, Δf and ΔD follow the linear density and viscosity dependency on temperature. Therefore, plotting the change in frequency over the change in time (i.e. the first derivative) versus the temperature, the under layer dependency on temperature for frequency data will be removed. This enables the observation of the thermoresponsive behavior independently from solvent density and viscosity changes. Such a plot is presented in Fig. 2c, where three cooling/heating cycles applied to the system are shown. Clearly, the switching is fully reversible, showing mean peak frequencies at 20.5 °C for cooling ramps and 24.6 °C for heating ramps. Taking the hysteresis (coming from the time constant of the system) into account, the average of these two mean values is representative of a steady state measurement, what gives a frequency peak at 22.6 °C, in good agreement with a reported transition temperature of 24.6 °C for brushes with a similar composition grown on SiO2 surfaces.29 Fig. 2d schematically shows the two steps collapse. These structural changes create new interfaces that modulate the interfacial viscosity.
Electrochemistry of FcCOOH and barrier effect of the polymer film
We performed the electrochemical oxidation of ferrocene carboxylic acid (FcCOOH) as a function of temperature in order to investigate the barrier effect of the polymer film towards electron transfer and diffusion. Since the transition temperature determined by E-QCM-D measurements is close to 22.6 °C, the temperature was varied between 10 and 47 °C. Fig. 3a shows the peak separation (ΔEp) and the ratio of anodic peak current over the reference peak current in a fully swollen state at 10 °C ( ) as a function of temperature for both a bare gold electrode and PME. Comparison of the reference temperature dependency on bare gold with PME demonstrates that ΔEp and r differ strongly for the two electrodes. For both parameters, values shift continuously in the temperature range of 10 to about 40 °C, where a plateau value is reached, in the case of PME, indicating that both the kinetics for electron transfer and diffusion issues are greatly affected by the polymer phase transition. ΔEp and r are linearly evolving till reaching a plateau, corresponding to the fully collapsed state, indicating a gradual thermal collapse. This finding is in agreement with temperature dependent thickness studies of similar surface brushes of poly(OEGMA-co-MEO2MA) copolymers.30 EIS was performed at several temperatures (Fig. 3b) ranging from 10 °C (fully swollen polymer) to 47 °C (fully collapsed polymer). Fig. 3c shows the RCT dependency on temperature. The temperature of 22.6 °C, where the collapsing was observed to be the most intense from E-QCM-D measurements, corresponds to the largest changes in RCT. RCT is no more strongly affected at temperatures above 30–33 °C. This corresponds to the beginning of zone 4 in Fig. 2a and b, where the polymer is considered to approach the maximum collapsed state. Finally, an 86% decrease of RCT is observed between fully swollen state (10 °C) and collapsed state (47 °C). This spectacular evolution of RCT points out the strong barrier effect of the swollen polymer.
) as a function of temperature for both a bare gold electrode and PME. Comparison of the reference temperature dependency on bare gold with PME demonstrates that ΔEp and r differ strongly for the two electrodes. For both parameters, values shift continuously in the temperature range of 10 to about 40 °C, where a plateau value is reached, in the case of PME, indicating that both the kinetics for electron transfer and diffusion issues are greatly affected by the polymer phase transition. ΔEp and r are linearly evolving till reaching a plateau, corresponding to the fully collapsed state, indicating a gradual thermal collapse. This finding is in agreement with temperature dependent thickness studies of similar surface brushes of poly(OEGMA-co-MEO2MA) copolymers.30 EIS was performed at several temperatures (Fig. 3b) ranging from 10 °C (fully swollen polymer) to 47 °C (fully collapsed polymer). Fig. 3c shows the RCT dependency on temperature. The temperature of 22.6 °C, where the collapsing was observed to be the most intense from E-QCM-D measurements, corresponds to the largest changes in RCT. RCT is no more strongly affected at temperatures above 30–33 °C. This corresponds to the beginning of zone 4 in Fig. 2a and b, where the polymer is considered to approach the maximum collapsed state. Finally, an 86% decrease of RCT is observed between fully swollen state (10 °C) and collapsed state (47 °C). This spectacular evolution of RCT points out the strong barrier effect of the swollen polymer.
Conclusions
We demonstrate that thermoresponsive brushes made of poly(OEGMA-co-MEO2MA) can be grown by surface initiated ATRP from gold substrates stepwise modified by a covalently attached polyamine layer and subsequent layer-by-layer (LBL) deposition of PSS, PDADMAC and of a polyelectrolyte macroinitiator as top layer. The modular character of the nevertheless rather simple built-up procedure allows for versatile and modular functionalizations of such polymer modified electrodes. As determined by electrochemical quartz crystal microbalance with dissipation (E-QCM-D), the film shows reversible switching between the swollen and the collapsed states by changing the temperature around the lower critical solution temperature (LCST). The dissipation profile with temperature suggests a two steps mechanism of the transition. This is confirmed by electrochemical oxidation of ferrocene carboxylic acid (FcCOOH) using the PME at different temperatures. The charge transfer resistance is gradually decreased with ongoing dehydration of the polymer brush while temperature is raised, till reaching a constant value when the polymer is fully collapsed. We believe that this large change of charge transfer resistance associated to the polymer state can be further implemented into switchable electrochemical systems.Acknowledgements
We gratefully acknowledge financial support from the Bundesministerium für Bildung und Forschung BMBF (German Federal Ministry of Education and Research), Initiative “Spitzenforschung & Innovation in den neuen Ländern” (“Das Taschentuchlabor”, FKZ 03IS2201B).References
- P. Schattling, F. D. Jochum and P. Theato, Polym. Chem., 2014, 5, 25–36 RSC.
- L. Zhai, Chem. Soc. Rev., 2013, 42, 7148–7160 RSC.
- I. Tokarev, M. Motornov and S. Minko, J. Mater. Chem., 2009, 19, 6932–6948 RSC.
- R. Barbey, L. Lavanant, D. Paripovic, N. Schüwer, C. Sugnaux, S. Tugulu and H.-A. Klok, Chem. Rev., 2009, 109, 5437–5527 CrossRef CAS PubMed.
- T. K. Tam, M. Ornatska, M. Pita, S. Minko and E. Katz, J. Phys. Chem. C, 2008, 112, 8438–8445 CAS.
- M. Privman, T. K. Tam, V. Bocharova, J. Halámek, J. Wang and E. Katz, ACS Appl. Mater. Interfaces, 2011, 3, 1620–1623 CAS.
- J.-F. Lutz, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3459–3470 CrossRef CAS PubMed.
- J.-F. Lutz, Ö. Akdemir and A. Hoth, J. Am. Chem. Soc., 2006, 128, 13046–13047 CrossRef CAS PubMed.
- K. Bebis, M. W. Jones, D. M. Haddleton and M. I. Gibson, Polym. Chem., 2011, 2, 975–982 RSC.
- J. Weiss, A. Li, E. Wischerhoff and A. Laschewsky, Polym. Chem., 2012, 3, 352–361 RSC.
- S. Inal, L. Chiappisi, J. D. Kölsch, M. Kraft, M.-S. Appavou, U. Scherf, M. Wagner, M. R. Hansen, M. Gradzielski, A. Laschewsky and D. Neher, J. Phys. Chem. B, 2013, 117, 14576–14587 CrossRef CAS PubMed.
- J.-F. Lutz, K. Weichenhan, Ö. Akdemir and A. Hoth, Macromolecules, 2007, 40, 2503–2508 CrossRef CAS.
- H. Kitano, T. Hirabayashi, M. Gemmei-Ide and M. Kyogoku, Macromol. Chem. Phys., 2004, 205, 1651–1659 CrossRef CAS PubMed.
- J.-F. Lutz and A. Hoth, Macromolecules, 2005, 39, 893–896 CrossRef.
- A. M. Jonas, K. Glinel, R. Oren, B. Nysten and W. T. S. Huck, Macromolecules, 2007, 40, 4403–4405 CrossRef CAS.
- A. Fandrich, J. Buller, E. Wischerhoff, A. Laschewsky and F. Lisdat, ChemPhysChem, 2012, 13, 2020–2023 CrossRef CAS PubMed.
- H. Dautzenberg, E. Görnitz and W. Jaeger, Macromol. Chem. Phys., 1998, 199, 1561–1571 CrossRef CAS.
- K. A. Marx, Biomacromolecules, 2003, 4, 1099–1120 CrossRef CAS PubMed.
- C. Kepplinger, F. Lisdat and U. Wollenberger, Langmuir, 2011, 27, 8309–8315 CrossRef CAS PubMed.
- P. Bertrand, A. Jonas, A. Laschewsky and R. Legras, Macromol. Rapid Commun., 2000, 21, 319–348 CrossRef CAS.
- G. Decher, in Multilayer Thin Films, Wiley-VCH Verlag GmbH & Co. KGaA, 2003, pp. 1–46 Search PubMed.
- E. Wischerhoff, K. Uhlig, A. Lankenau, H. G. Börner, A. Laschewsky, C. Duschl and J.-F. Lutz, Angew. Chem., Int. Ed., 2008, 47, 5666–5668 CrossRef CAS PubMed.
- E. Wischerhoff, S. Glatzel, K. Uhlig, A. Lankenau, J.-F. Lutz and A. Laschewsky, Langmuir, 2009, 25, 5949–5956 CrossRef CAS PubMed.
- S. Edmondson and S. P. Armes, Polym. Int., 2009, 58, 307–316 CrossRef CAS PubMed.
- N. C. Estillore and R. C. Advincula, Macromol. Chem. Phys., 2011, 212, 1552–1566 CrossRef CAS PubMed.
- D. Johannsmann, Macromol. Chem. Phys., 1999, 200, 501–516 CrossRef CAS.
- D. Johannsmann, Phys. Chem. Chem. Phys., 2008, 10, 4516–4534 RSC.
- M. V. Voinova, M. Rodahl, M. Jonson and B. Kasemo, Phys. Scr., 1999, 59, 391 CrossRef CAS.
- X. Laloyaux, B. Mathy, B. Nysten and A. M. Jonas, Langmuir, 2010, 26, 838–847 CrossRef CAS PubMed.
- J. Buller, A. Laschewsky and E. Wischerhoff, Soft Matter, 2013, 9, 929–937 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Covalent coupling of PEI onto DTSP and dissipation shift during LBL assembly. See DOI: 10.1039/c4ra07190e |
| ‡ Permanent address, Université de Poitiers, UFR SFA - Bâtiment B27, 4 rue Michel Brunet, TSA 51106, 86073 Poitiers cedex 9, France. Fax: +33 5 49453580; Tel: +33 5 49453628; E-mail: E-mail: clement.comminges@univ-poitiers.fr |
| This journal is © The Royal Society of Chemistry 2014 |