Preparation of magnetic mesoporous core–shell nanocomposites for cinnamic acid hydrogenation†
Xiaofang Liu,
Lingjuan Shi,
Wenhui Feng,
Libo Niu,
Chen Liu and
Guoyi Bai*
Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of Ministry of Education, College of Chemistry and Environmental Science, Hebei University, Baoding 071002, P.R. China. E-mail: baiguoyi@hotmail.com; Fax: +86-312-5937102; Tel: +86-312-5079359
First published on 11th September 2014
Abstract
A novel magnetic mesoporous core–shell nanocomposite Fe3O4@nSiO2@mSiO2@Ni–Co–B was prepared by the combination of a modified Stöber sol–gel process, a surfactant-templating method and self-assembly. It has a multi-shell structure with ferroferric oxide as core, dense nonporous silica and mesoporous silica as middle layers in sequence and an Ni–Co–B amorphous alloy as outer layer, as confirmed by transmission electron microscopy and nitrogen adsorption–desorption. This nanocomposite showed high activity and good selectivity in the selective hydrogenation of cinnamic acid to hydrocinnamic acid. Notably, it can be easily separated by a magnet after reaction due to its high magnetism and recycled effectively five times.
1. Introduction
In recent years, magnetic core–shell silica nanocomposites, which combine the functionalities of magnetic cores and silica shells, have attracted much attention not only due to their unique structures but also their wide applications in the fields of drug delivery, catalysis, environment protection, bioseparation and so on.1–7 For example, Lin et al. have prepared a multifunctional Fe3O4@nSiO2@mSiO2@YVO4:Eu3+ composite material, which possesses ordered hexagonal mesopores, bright luminescence, high magnetization saturation value, and can be potentially used as targeted drug delivery system.4 Zhao et al. have prepared Fe3O4@SiO2–Au@mSiO2 microspheres, which showed high conversion and selectivity in the catalytic reduction of 4-nitrophenol and styrene epoxidation.5 Zhang et al. have designed an effective Fe3O4@SiO2@mSiO2–Pd(0) catalyst for Suzuki coupling reaction.6 However, most of these catalysts applied noble metals as active components, and the high price of noble metals limited their further applications.On the other hand, Ni–B amorphous alloy catalysts have attracted much interest in hydrogenation reactions owing to their unique isotropic nature, high concentration of coordinative unsaturated sites, and low cost.8–12 However, most of the pure Ni–B amorphous alloys showed either poor stability or activity. Thus, great efforts have been attempted to solve these problems. For example, Liu et al. have reported a supported Ni–B/boehmite catalyst, which exhibited good activity and stability in the hydrogenation of p-nitrophenol and p-chloronitrobenzene.9 Recently, we have prepared a Fe3O4@nSiO2@Ni–La–B core–shell catalyst and obtained good results in the hydrogenation of benzophenone.10 However, due to its low surface area, large amounts of active components and catalyst were applied in that reaction. Thus, there is still a need to develop a more efficient catalyst with higher surface area and lower active components loadings. Mesoporous materials, which have high surface area and large pore volume, can facilitate the loading of the active components and might be a good choice to solve this problem.
Very recently, we have prepared a NiCoB/γ-Al2O3-u catalyst, which showed both suitable activity and stability in tert-butanol, in the selective hydrogenation of cinnamic acid to hydrocinnamic acid,11 an important chemical intermediate.13,14 But the used catalyst was separated by leaching for recycling, not as effective as magnetic separation. Combined with the results of our previous work10–12 and the structural benefit of mesoporous materials, we designed a novel Fe3O4@nSiO2@mSiO2@Ni–Co–B (n: nonporous, m: mesoporous) magnetic mesoporous nanocomposite with four layer core–shell structure and evaluated it in the selective hydrogenation of cinnamic acid to hydrocinnamic acid. As envisaged, Fe3O4@nSiO2@mSiO2@Ni–Co–B showed high activity, good selectivity and stability under relatively mild reaction conditions. Meanwhile, it can be easily separated from the reaction mixture by magnet and effectively recycled.
2. Experimental
2.1. Preparation of catalysts
Unless otherwise stated, all chemicals were purchased from Baoding Huaxin Reagent and Apparatus Co., Ltd. and were used as received without further purification. The Fe3O4@nSiO2@mSiO2@Ni–Co–B nanocomposite was prepared by a multistep procedure, as shown in Scheme 1. Fe3O4@nSiO2@mSiO2 was first prepared according to the method similar to the literature15 (for details, see the ESI†). Then, 0.5 g Fe3O4@nSiO2@mSiO2 was impregnated with an aqueous solution of NiCl2·6H2O (0.506 g) and CoCl2·6H2O (0.051 g) (molar ratio Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co = 10
Co = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After drying at 313 K, the precursor was reduced by adding 8.6 mL of 1.0 M aqueous KBH4 containing 0.2 M NaOH dropwise in an ice water bath under ultrasound. After the reduction was finished, the precipitate was first separated using magnet and then washed with deionized water several times until neutral, followed by washing with absolute ethanol three times to remove the residual water. Finally, the obtained Fe3O4@nSiO2@mSiO2@Ni–Co–B nanocomposite was kept under absolute ethanol for further use. Fe3O4@nSiO2@Ni–Co–B, Ni–Co–B, and Ni–Co–B/mSiO2 were prepared in a similar manner to the protocol reported in our previous work.10–12
1). After drying at 313 K, the precursor was reduced by adding 8.6 mL of 1.0 M aqueous KBH4 containing 0.2 M NaOH dropwise in an ice water bath under ultrasound. After the reduction was finished, the precipitate was first separated using magnet and then washed with deionized water several times until neutral, followed by washing with absolute ethanol three times to remove the residual water. Finally, the obtained Fe3O4@nSiO2@mSiO2@Ni–Co–B nanocomposite was kept under absolute ethanol for further use. Fe3O4@nSiO2@Ni–Co–B, Ni–Co–B, and Ni–Co–B/mSiO2 were prepared in a similar manner to the protocol reported in our previous work.10–12
2.2. Catalyst characterization
X-ray diffraction (XRD) patterns were obtained on a Bruker D8 diffractometer using a Cu Kα radiation source. The Fourier transform infrared spectra (FT-IR) were tested on a Bruker VERTEX 70 Fourier transform spectrophotometer using KBr pellets. Transmission electron microscopy (TEM), selected-area electron diffraction (SAED) and X-ray energy dispersive spectroscopy (EDS) were carried out on a microscope JEM-2100F instrument. BET surface area and pore volume of the nanocomposites were measured using a Micromeritics Tristar II 3020 surface area and pore analyzer. Magnetization curves were performed on a LDJ9600-1 Superconducting quantum interference device at room temperature.2.3. Activity test
Selective hydrogenation of cinnamic acid (Scheme 2) was performed as follows: cinnamic acid (3.0 g), catalyst (0.1 g) and tert-butanol (200 mL) were mixed in a 500 mL stainless steel autoclave equipped with a mechanical stirrer and electric heating system. The autoclave was filled with H2 three times to exclude residual air and then pressurized to 3.0 MPa with H2. After it was heated to 373 K, hydrogenation was started by stirring the reaction mixture vigorously and allowed to proceed for 1 h. Reaction mixtures were analyzed by gas chromatography using a 30 m SE-30 capillary column and the product structures were identified using gas chromatography-mass spectrometry (GC-MS) on an Agilent 5975C spectrometer. The used catalyst was separated by magnet, washed with tert-butanol and then kept under tert-butanol for recycling.3. Results and discussion
Fig. 1A shows the wide-angle XRD patterns of Fe3O4, Fe3O4@nSiO2@mSiO2, Fe3O4@nSiO2@mSiO2@Ni–Co–B, and Fe3O4@nSiO2@Ni–Co–B. The characteristic diffraction peaks of these magnetic core–shell nanocomposites are just like those of pure Fe3O4, which possess a face-centered cubic structural (Fd3m space group) magnetite (JCPDS no. 19-0629), indicating that the magnetic cores were well retained after the silica coating.16 Furthermore, the broad band at 2θ = 20–30° in Fe3O4@nSiO2@mSiO2, Fe3O4@nSiO2@mSiO2@Ni–Co–B, and Fe3O4@nSiO2@Ni–Co–B can be assigned to the amorphous SiO2, suggesting that both the nSiO2 and mSiO2 shells existed in an amorphous state.4 In contrast, no characteristic diffraction peaks related to Ni–Co–B amorphous alloy can be observed at about 2θ = 45° in Fe3O4@nSiO2@Ni–Co–B and Fe3O4@nSiO2@mSiO2@Ni–Co–B. Fortunately, both Ni and Co can be detected by EDS on the surface of Fe3O4@nSiO2@mSiO2@Ni–Co–B (Fig. 1B) and its amorphous structure can be further confirmed by a successive diffraction halo in the SAED image (Fig. 1B inset).17 Thus, the amorphous Ni–Co–B was suggested to be well dispersed on the surface of the mSiO2 shell.FT-IR spectra of Fe3O4@nSiO2@CTAB/SiO2 without the treatment of ethanol containing NH4NO3 and the obtained Fe3O4@nSiO2@mSiO2 nanocomposites are presented in Fig. 2. The adsorption peak appearing at about 571 cm−1 is assigned to the characteristic absorption peak of Fe–O bond, indicating the presence of Fe3O4 magnetic nanaoparticles. The absorption peaks at 1075 cm−1 and 792 cm−1, 945 cm−1, 457 cm−1 correspond to Si–O–Si, Si–OH, Si–O respectively, demonstrating the formation of SiO2.18 For the Fe3O4@nSiO2@CTAB/SiO2, the bands observed in the region of 2800–3000 cm−1 are attributed to the vibrations of –CH2 of CTAB templates. In contrast, no adsorption peaks were observed in the range of 2800–3000 cm−1 for the Fe3O4@nSiO2@mSiO2 nanocomposites, suggesting that the CTAB templates have been completely removed after the treatment with ethanol containing NH4NO3.16
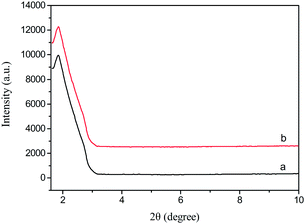
The low-angle XRD patterns of Fe3O4@nSiO2@mSiO2 and Fe3O4@nSiO2@mSiO2@Ni–Co–B are shown in Fig. 3. The sole diffraction peak at about 2θ = 2° for both the samples indicated the long-range ordered structures of them. Considering the high similarity of these two low-angle XRD patterns, we could suggest that the uniform mesoporous structures were maintained after loading of Ni–Co–B.19
TEM images of Fe3O4@nSiO2@mSiO2 and Fe3O4@nSiO2@mSiO2@Ni–Co–B are exhibited in Fig. 4. As can be seen, Fe3O4@nSiO2@mSiO2 nanocomposite exhibits a clear multi-shell structure with a magnetite core, a nonporous silica shell in the middle, and a mesoporous silica shell outside (Fig. 4a), although certain aggregation occurred due to the inherent magnetism of magnetite. The magnetite core is approximately 100 nm in diameter, the inner nonporous silica shell is approximately 20 nm and the mesoporous silica shell is approximately 45 nm with some mesoporous channels (as indicated by the arrows in Fig. 4b). In contrast, Fe3O4@nSiO2@mSiO2@Ni–Co–B keeps a typical multi-shell structure (Fig. 4c and d), but not as clear as Fe3O4@nSiO2@mSiO2 after the loading of Ni–Co–B, implying the Ni–Co–B amorphous alloy being dispersed on the outer surface or in the pores of mSiO2.
Fig. 5 exhibits the nitrogen adsorption–desorption isotherms and pore size distribution curves of Fe3O4@nSiO2@mSiO2, Fe3O4@nSiO2@mSiO2@Ni–Co–B and Fe3O4@nSiO2@Ni–Co–B. The nitrogen sorption isotherms of Fe3O4@nSiO2@mSiO2 resembled a reversible IV-type isotherm20 with nearly no hysteresis loop (Fig. 5A(a)), mainly due to the reversible pore filling in the P/P0 range of 0.2–0.4 as a form of capillary condensation.21 In contrast, Fe3O4@nSiO2@mSiO2@Ni–Co–B exhibited a IV-type isotherm with an obvious hysteresis loop, which is likely to be associated with some interstitial structures form among the aggregated particles in the sample.22 Their pore size distribution curves (Fig. 5B(a) and (b)) presented a sharp peak at about 2.3 nm, which verified their mesoporous structure.23 In contrast, the nitrogen sorption isotherms of Fe3O4@nSiO2@Ni–Co–B showed III-type isotherm24 (Fig. 5A(c)) and its pore size distribution curve merely fluctuated slightly over a wide range (Fig. 5B(c)), indicating its nearly non-porous structure.
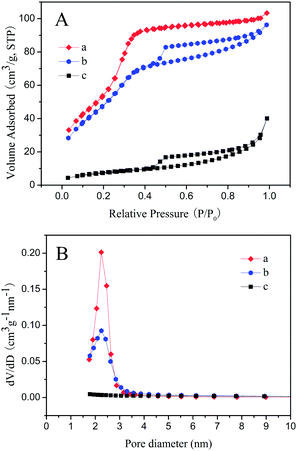 | ||
| Fig. 5 (A) N2 adsorption–desorption isotherms and (B) pore size distribution curves of (a) Fe3O4@nSiO2@mSiO2, (b) Fe3O4@nSiO2@mSiO2@Ni–Co–B and (c) Fe3O4@nSiO2@Ni–Co–B. | ||
The results of BET surface area and pore volume of the core–shell nanocomposites and Ni–Co–B are summarized in Table 1. It was found that the surface area markedly increased from 5.1 to 302.8 m2 g−1 and the pore volume from 0.009 to 0.186 cm3 g−1 after coating an mSiO2 shell outside of Fe3O4@nSiO2, also demonstrating the mesoporous structure of Fe3O4@nSiO2@mSiO2. Considering that the BET surface area and pore volume of Fe3O4@nSiO2@mSiO2@Ni–Co–B decreased to 200.0 m2 g−1 and 0.145 cm3 g−1 respectively, we speculated that Ni–Co–B has dispersed on the outer surface and occupied some pores of Fe3O4@nSiO2@mSiO2.25 However, these values of Fe3O4@nSiO2@mSiO2@Ni–Co–B are still much higher than those of Fe3O4@nSiO2@Ni–Co–B, ascribed to the presence of mSiO2.
| Sample | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|
| a Reaction conditions: 3.0 g cinnamic acid, 0.1 g catalyst (20 wt% Ni–Co–B), 200 mL tert-butanol, temperature at 373 K, initial P(H2) = 3.0 MPa, and reaction time 1 h.b Ni–Co–B 0.02 g. | ||||
| Fe3O4@nSiO2 | 5.1 | 0.009 | — | — |
| Fe3O4@nSiO2@mSiO2 | 302.8 | 0.186 | — | — |
| Fe3O4@nSiO2@Ni–Co–B | 27.0 | 0.062 | 58.4 | 100.0 |
| Fe3O4@nSiO2@mSiO2@Ni–Co–B | 200.0 | 0.145 | 100.0 | 100.0 |
| Ni–Co–Bb | 21.9 | 0.064 | 65.9 | 100.0 |
The room temperature hysteresis loops of Fe3O4@nSiO2@mSiO2@Ni–Co–B nanocomposite and Ni–Co–B amorphous alloy at an applied field of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe are shown in Fig. 6. As can be seen, the saturation magnetization (Ms) value is 8.83 emu g−1 for Fe3O4@nSiO2@mSiO2@Ni–Co–B and no remanence or hysteresis loops are detectable, indicating its superparamagnetism;26 whereas, the Ms value of Ni–Co–B is only 0.08 emu g−1. Obviously, due to its high Ms value, Fe3O4@nSiO2@mSiO2@Ni–Co–B can be easily separated for recycling by an external magnetic field; but the pure Ni–Co–B cannot (Fig. 6 inset).
000 Oe are shown in Fig. 6. As can be seen, the saturation magnetization (Ms) value is 8.83 emu g−1 for Fe3O4@nSiO2@mSiO2@Ni–Co–B and no remanence or hysteresis loops are detectable, indicating its superparamagnetism;26 whereas, the Ms value of Ni–Co–B is only 0.08 emu g−1. Obviously, due to its high Ms value, Fe3O4@nSiO2@mSiO2@Ni–Co–B can be easily separated for recycling by an external magnetic field; but the pure Ni–Co–B cannot (Fig. 6 inset).
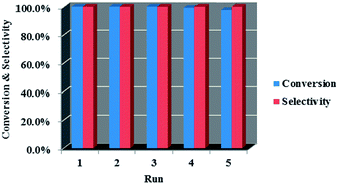
The catalytic performance of the two core–shell nanocomposites were tested in the hydrogenation of cinnamic acid and Ni–Co–B was also evaluated for comparison, and the results are listed in Table 1. As can be seen, the conversion of cinnamic acid was 100% over Fe3O4@nSiO2@mSiO2@Ni–Co–B, much higher than those of Fe3O4@nSiO2@Ni–Co–B (58.4%) and Ni–Co–B (65.9%), although the selectivity for hydrocinnamic acid were 100% for all catalysts. We ascribed this to the effect of the mSiO2 shell, which can not only benefit the self-assembly of Ni–Co–B by modulating the surface negatively charged,27 but also promote the dispersion of Ni–Co–B nanoparticle on its mesoporous surface. The good catalytic performance of Ni–Co–B/mSiO2 can support this assumption, which also shows 100% conversion and selectivity in the hydrogenation of cinnamic acid. However, it must be separated by centrifugation, which is not as convenient as magnetic separation of Fe3O4@nSiO2@mSiO2@Ni–Co–B. Furthermore, we think the low BET surface area (5.1 m2 g−1) and pore volume (0.009 cm3 g−1) of Fe3O4@nSiO2 should account for the lower activity of Fe3O4@nSiO2@Ni–Co–B, compared to Ni–Co–B. Finally, the stability of Fe3O4@nSiO2@mSiO2@Ni–Co–B was investigated in the hydrogenation of cinnamic acid and the results were depicted in Fig. 7. As expected, this core–shell nanocomposite can be easily separated by magnet and recycled five times without significant loss of its initial catalytic activity with the selectivity for hydrocinnamic acid still keeping 100%.
4. Conclusions
In conclusion, a novel magnetic mesoporous core–shell nanocomposite Fe3O4@nSiO2@mSiO2@Ni–Co–B was prepared and showed excellent activity in the selective hydrogenation of cinnamic acid to hydrocinnamic acid. The existence of the mSiO2 shell affords this nanocomposite larger surface area and pore volume, making the active Ni–Co–B species highly dispersed on its surface, and then accounting for its high activity. Meanwhile, this nanocomposite can be easily separated by magnet after reaction due to its high magnetism and recycled five times without significant loss of its initial catalytic activity, demonstrating its good stability.Acknowledgements
Financial support by the National Natural Science Foundation of China (21376060), the Natural Science Foundation of Hebei Province (B2014201024), and the Science Foundation of Hebei University (3333112) are gratefully acknowledged.Notes and references
- Z. H. Xu, C. X. Li, X. J. Kang, D. M. Yang, P. P. Yang, Z. Y. Hou and J. Lin, J. Phys. Chem. C, 2010, 114, 16343–16350 CAS.
- J. P. Ge, Q. Zhang, T. R. Zhang and Y. D. Yin, Angew. Chem., Int. Ed., 2008, 47, 8924–8928 CrossRef CAS PubMed.
- D. G. He, X. X. He, K. M. Wang, Y. X. Zhao and Z. Zou, Langmuir, 2013, 29, 5896–5904 CrossRef CAS PubMed.
- P. P. Yang, Z. W. Quan, Z. Y. Hou, C. X. Li, X. J. Kang, Z. Y. Cheng and J. Lin, Biomaterials, 2009, 30, 4786–4795 CrossRef CAS PubMed.
- Y. H. Deng, Y. Cai, Z. K. Sun, J. Liu, C. Liu, J. Wei, W. Li, C. Liu, Y. Wang and D. Y. Zhao, J. Am. Chem. Soc., 2010, 132, 8466–8473 CrossRef CAS PubMed.
- W. Li, B. L. Zhang, X. J. Li, H. P. Zhang and Q. Y. Zhang, Appl. Catal., A, 2013, 459, 65–72 CrossRef CAS PubMed.
- H. M. Chen, S. S. Liu, H. L. Yang, Y. Mao, C. H. Deng, X. M. Zhang and P. Y. Yang, Proteomics, 2010, 10, 930–939 CAS.
- J. B. Zheng, Z. Q. Xia, J. J. Li, W. K. Lai, X. D. Yi, B. H. Chen, W. P. Fang and H. L. Wan, Catal. Commun., 2012, 21, 18–21 CrossRef CAS PubMed.
- H. Liu, J. Deng and W. Li, Catal. Lett., 2010, 137, 261–266 CrossRef CAS.
- G. Y. Bai, L. J. Shi, Z. Zhao, Y. L. Wang, M. D. Qiu and H. X. Dong, Mater. Lett., 2013, 96, 93–96 CrossRef CAS PubMed.
- G. Y. Bai, H. X. Dong, Z. Zhao, H. L. Chu, X. Wen, C. Liu and F. Li, RSC Adv., 2014, 4, 19800–19805 RSC.
- G. Y. Bai, H. X. Dong, Z. Zhao, Y. L. Wang, Q. Z. Chen and M. D. Qiu, J. Nanosci. Nanotechnol., 2013, 13, 5012–5016 CrossRef CAS PubMed.
- K. Lan and Z. X. Shan, Synth. Commun., 2007, 37, 2171–2177 CrossRef CAS.
- K. J. P. Narayana, P. Prabhakar, M. Vijayalakshmi, Y. Venkateswarlu and P. S. J. Krishna, Pol. J. Microbiol., 2007, 56, 191–197 CAS.
- Y. H. Deng, D. W. Qi, C. H. Deng, X. M. Zhang and D. Y. Zhao, J. Am. Chem. Soc., 2008, 130, 28–29 CrossRef CAS PubMed.
- C. Wang, S. Y. Tao, W. Wei, C. G. Meng, F. Y. Liu and M. Han, J. Mater. Chem., 2010, 20, 4635–4641 RSC.
- H. Li, J. Liu, H. X. Yang and H. Li, J. Mater. Res., 2009, 24, 3300–3307 CrossRef CAS.
- S. Z. Li, Y. Ma, X. L. Yue, Z. Cao and Z. F. Dai, New J. Chem., 2009, 33, 2414–2418 RSC.
- X. H. Zhu, Y. Z. Chen, F. W. Zhang, J. R. Niu, M. Xie and J. T. Ma, RSC Adv., 2014, 4, 2509–2514 RSC.
- J. P. Yang, F. Zhang, Y. R. Chen, S. Qian, P. Hu, W. Li, Y. H. Deng, Y. Fang, L. Han, M. Luqmanb and D. Y. Zhao, Chem. Commun., 2011, 47, 11618–11620 RSC.
- P. J. Branton, K. Kaneko and N. Setoyama, Langmuir, 1996, 12, 599–600 CrossRef CAS.
- S. S. Liu, H. M. Chen, X. H. Lu, C. H. Deng, X. M. Zhang and P. Y. Yang, Angew. Chem., Int. Ed., 2010, 49, 7557–7561 CrossRef CAS PubMed.
- Z. N. Liu, H. H. Yang, H. Zhang, C. J. Huang and L. F. Li, Cryogenics, 2012, 52, 699–703 CrossRef CAS PubMed.
- H. F. Liu, S. F. Ji, H. Yang, H. Zhang and M. Tang, Ultrason. Sonochem., 2014, 21, 505–512 CrossRef CAS PubMed.
- R. Xu, G. Y. Sun, Q. Y. Li, E. B. Wang and J. M. Gu, Solid State Sci., 2010, 12, 1720–1725 CrossRef CAS PubMed.
- X. H. Zhang and L. Jiang, J. Mater. Chem., 2011, 21, 10653–10657 RSC.
- D. W. Wang, X. M. Zhu, S. F. Lee, H. M. Chan, H. W. Li, S. K. Kong, J. C. Yu, C. H. K. Cheng, Y. X. J. Wang and K. C. F. Leung, J. Mater. Chem. B, 2013, 1, 2934–2942 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07176j |
| This journal is © The Royal Society of Chemistry 2014 |