Development of a novel one-pot synthetic method for the preparation of (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites and the study of their microwave absorption and magnetic properties†
Subhenjit Hazraa,
Barun Kumar Ghosha,
Hrishikesh Ravindra Joshia,
Manoj Kumar Patrab,
Raj Kumar Janib,
Sampat Raj Vaderab and
Narendra Nath Ghosh*a
aNano-Materials Lab, Department of Chemistry, Birla Institute of Technology and Science, Pilani KK Birla Goa Campus, Zuarinagar, Goa 403726, India. E-mail: naren70@yahoo.com; Fax: +91 832 2557033; Tel: +91 832 2580318
bDefence Lab, Jodhpur 342011, India
First published on 5th September 2014
Abstract
The development of a simple yet novel aqueous solution based ‘one-pot’ method has been reported for the preparation of nanocomposites composed of soft ferrite (Mn0.2Ni0.4Zn0.4Fe2O4) and hard ferrite (BaFe12O19) phases. A physical mixing method has also been employed to prepare nanocomposites having the same compositions. The effects of synthetic methodologies on the microstructures of the nanocomposites as well as their magnetic and microwave absorption properties have been evaluated. The crystal structures and microstructures of these composites have been investigated using X-ray diffraction, transmission electron microscopy and scanning electron microscopy. In the nanocomposites prepared by both methods, the presence of nanocrystalline Mn0.2Ni0.4Zn0.4Fe2O4 and BaFe12O19 phases were detected. However, nanocomposites prepared by the one-pot method possessed better homogeneous distribution of hard and soft ferrite phases than the nanocomposites prepared by the physical mixing method. Very good spring exchange coupling interaction between the hard and soft ferrite phases was observed for the nanocomposites prepared by the one-pot method and these composites exhibited magnetically single phase behaviour. The spring exchange coupling interaction enhanced the magnetic properties (high saturation magnetization and coercivity) and microwave absorption properties of the nanocomposites prepared by the one-pot method, in comparison with the nanocomposites prepared by the physical mixing method as well as pure Mn0.2Ni0.4Zn0.4Fe2O4 and BaFe12O19 nanoparticles. The minimum reflection loss of the composites was found to be ∼−25 dB (i.e. >99% absorption) at 8.2 GHz with an absorber thickness of 3.5 mm.
1. Introduction
Microwave-absorbing materials have gained immense interest from scientists and technologists due to their usage in military applications and commodity markets. Microwave-absorbing materials can be used in stealth defence systems because they can effectively reduce the radar cross-section of targets. These materials also have the potential to address issues related to environmental pollution caused by electromagnetic interference (EMI) due to the extensive use of electronic devices such as computer networks, and mobile phones.1–7 Hence, the reduction of interference from electromagnetic radiations, especially microwave radiation, is highly desirable in the current scenario. Therefore, there has been a great deal of interest in developing microwave radiation absorbing materials. The microwave absorbing materials, which operate in the frequency range of 8.2–12.4 GHz (X-band) and is also known as radar absorbing material (RAM), find a wide range of applications, including electronic devices and military equipment. Researchers are showing specific interest for the development of efficient RAM in the last few decades. Various types of materials such as dielectric, magnetic, conducting polymer and composites have been investigated to achieve RAMs with desired properties.8–11Ferrites are an important class of magnetic materials which exhibit microwave absorption property.12–19 Spinel ferrites are used in the megahertz range because of the Snoek's limitation rule, whereas hexaferrites exhibit microwave absorption in the gigahertz range, although the band width is narrow.20–22 However, it is difficult for a single material to fulfil all the requirements (such as large absorption peak, wide working frequency range and thin absorption layer) of an ideal radar absorber. Nanocomposites consisting of hard and soft ferrite phase can offer unified systems whose properties are complimentary or even mutually exclusive.
Recently, there has been immense attention on materials having good spring exchange coupling behaviour. According to the exchange spring theory proposed by Kneller and Hawig,23 nanocomposite magnets consisting of soft and hard phases with sufficient exchange coupling between both phases are known as exchange spring magnets. Exchange spring magnets possess high saturation magnetization of the soft phase and high coercivity of the hard phase, which leads to the production of large energy product values and are potential candidates for next generation permanent magnets.24 These materials also exhibit improved microwave absorbing properties.1,2,25,26 According to Maeda et al.,27 the exchange interaction between hard and soft ferrite phases can enhance the microwave absorption properties. The exchange spring behaviours of some metal alloy systems and multilayer systems (such as Pr2Fe12B,28 Nd–Fe–B,29 Sm–Co/Fe,30 SmCox–Co,31 BaCo2Fe16O27 or Ba2Co2Fe12O22–FeCo alloy,32 and BaFe12O19/α-Fe33) have been reported by researchers. Compared to metallic systems, nanocomposites composed of soft spinel ferrite and hard hexagonal ferrite have shown their potential to be a promising candidate for superior permanent magnets because of their low cost, excellent corrosion resistance behaviour and high electrical resistivity. However, to the best of our knowledge, reports on soft–hard ferrite nanocomposites are very limited in the literature1,2,24,34–38 due to the lack of availability of simple preparation techniques. Most of the reported methods are complex in nature and require high sintering temperatures. Roy et al. reported the preparation of NiZn ferrite–barium ferrite composite, where NiZn ferrite and barium ferrite were prepared separately by co-precipitation method and high temperature solid state method (sintered at 1200 °C) respectively. NiZn ferrite and barium ferrite powders were then mixed in appropriate weight ratios and heat treated at different temperatures ranging from 400 to 800 °C.35 They have also reported the preparation of BaCa2Fe16O27–Fe3O4 composites, where BaCa2Fe16O27 was prepared by citrate method and commercial grade Fe3O4 was procured.34 Chen et al.26 employed citrate based sol–gel technique to synthesize composite powders with different weight ratios of strontium hexaferrite to zinc ferrite and studied their microwave absorption properties. Song et al.24 reported a sol–gel citrate route for the preparation of SrFe12O19/Ni0.5Zn0.5Fe2O4 hollow microfiber at 900 °C. The synthesis and microwave absorbing properties of nanocomposites consisting of BaFe12O19 (BFO)/Ni0.5Zn0.5Fe2O4 (NZFO) ferrite microfibers with different mass ratios was investigated by Shen et al.1 Xie et al.38 investigated Sr–Zn ferrite composites, which were synthesized by chemical coprecipitation with a two-step sintering process at 700 °C for 6 h and then 1000 °C for 2 h. Tyagi et al.2 reported a composite consisting of strontium ferrite/Ni–Zn ferrite powders prepared by co-precipitation method and they also studied its microwave absorption properties in the X-band.
A high level of homogenous mixing of the hard and soft ferrite phases is required for the fabrication of exchange spring magnet. This is possible when both phases can be grown together from the same reaction mixture. In this paper, we are reporting a simple ‘one-pot’ synthetic route for the preparation of nanocomposites containing both Mn0.2Ni0.4Zn0.4Fe2O4 (soft ferrite)–BaFe12O19 (hard ferrite) phases with a wide range of compositions. Nanocomposites having the same compositions were also prepared by employing the ‘physical mixing’ method using pure Mn0.2Ni0.4Zn0.4Fe2O4 and BaFe12O19 nanopowders.
Magnetic and microwave absorption properties of both of these types of composites were measured and compared.
2. Experimental
2.1. Materials
BaCO3, Mn(NO3)2·4H2O, Ni(NO3)2·6H2O, Fe(NO3)3·9H2O, Zn dust, nitric acid and ethylenediaminetetraacetic acid (EDTA) were purchased from Merck, India and used without further purification. Zn(NO3)2 and Ba(NO3)2 were prepared by dissolving Zn dust and BaCO3, respectively, in aqueous nitric acid.2.2. Synthesis of (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites by one-pot method (OP method)
To prepare (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites (with x = 0.85, 0.75, 0.5 and 0.25) using one-pot method, a stoichiometric amount of BaNO3, Mn(NO3)2·4H2O, Ni(NO3)2·6H2O, Zn(NO3)2, Fe(NO3)3·9H2O, and EDTA aqueous solutions were mixed in a beaker (Table 1) and stirred for 2 h. This reaction mixture was then dried at ∼110 °C for 2 h. A black flaky carbonaceous material was formed after drying, which is referred as the precursor powder. Then, the precursor powders were calcined at 800 °C for 4 h in air to obtain pure (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites.| Composition | MnII-nitratea (g) | Zn dusta (g) | NiII-nitratea (g) | FeIII-nitratea (g) | Ba-carbonatea (g) | EDTAa (g) | Mn0.2Ni0.4Zn0.4F2O4b (g) | BaFe12O19b (g) |
|---|---|---|---|---|---|---|---|---|
| a For one-pot method.b For physical mixing method. | ||||||||
| Mn0.2Ni0.4Zn0.4Fe2O4-pure | 0.212 | 0.112 | 0.497 | 3.419 | — | 3.71 | — | — |
| (Mn0.2Ni0.4Zn0.4Fe2O4)0.85–(BaFe12O19)0.15 | 0.116 | 0.06 | 0.269 | 3.846 | 0.08 | 14.31 | 0.55 | 0.45 |
| (Mn0.2Ni0.4Zn0.4Fe2O4)0.75–(BaFe12O19)0.25 | 0.082 | 0.043 | 0.192 | 3.98 | 0.108 | 14.21 | 0.39 | 0.61 |
| (Mn0.2Ni0.4Zn0.4Fe2O4)0.5–(BaFe12O19)0.5 | 0.037 | 0.019 | 0.086 | 4.18 | 0.146 | 13.91 | 0.18 | 0.82 |
| (Mn0.2Ni0.4Zn0.4Fe2O4)0.25–(BaFe12O19)0.75 | 0.014 | 0.007 | 0.032 | 4.3 | 0.166 | 13.75 | 0.07 | 0.93 |
| BaFe12O19-pure | — | — | — | 4.365 | 0.177 | 13.74 | — | — |
2.3. Synthesis of (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites by physical mixing method (PM method)
We prepared a set of composite samples with various composition (with x = 0.85, 0.75, 0.5 and 0.25) by the ‘physical mixing’ method where pure Mn0.2Ni0.4Zn0.4Fe2O4 and BaFe12O19 powders were mixed with appropriate weight ratios (Table 1) using a mortar and pestle. Pure BaFe12O19 and Mn0.2Ni0.4Zn0.4Fe2O4 nanopowders were prepared separately by using the EDTA-precursor method, which is developed by us.39,40 For the synthesis of BaFe12O19, stoichiometric amounts of Ba(NO3)2 and Fe(NO3)3·9H2O were dissolved in distilled water according to the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, as shown in Table 1. An aqueous solution of EDTA was prepared by dissolving EDTA in hot water with the dropwise addition of NH4OH. After the complete dissolution of EDTA, the solution was boiled to remove the excess NH3. The pH of the solution was ∼6. Aqueous solutions of metal nitrates and EDTA were mixed in a molar ratio of 1
12, as shown in Table 1. An aqueous solution of EDTA was prepared by dissolving EDTA in hot water with the dropwise addition of NH4OH. After the complete dissolution of EDTA, the solution was boiled to remove the excess NH3. The pH of the solution was ∼6. Aqueous solutions of metal nitrates and EDTA were mixed in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and stirred for 1 h at room temperature using a magnetic stirrer.
4 and stirred for 1 h at room temperature using a magnetic stirrer.
The pH of the resulting mixture was ∼2. A black precursor was formed when the mixture was evaporated to dryness on a hot plate at ∼110 °C. The precursor powder was then calcined in air for 4 h at 800 °C to obtain BaFe12O19 nanopowder.
To prepare Mn0.2Ni0.4Zn0.4Fe2O4 nanopowder, we used metal nitrates, such as Fe(NO3)3·9H2O, Ni(NO3)2·6H2O, Mn(NO3)2·4H2O, and Zn(NO3)2 as starting materials and water as solvent. Stoichiometric amounts of metal nitrates were dissolved in distilled water according to the molar compositions as shown in Table 1. The aqueous solutions of metal nitrates and EDTA were mixed in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and stirred for 1 h at room temperature using a magnetic stirrer. The pH of the reaction mixture was found to be ∼2. Dark brown precursors were formed when the reaction mixtures were evaporated to dryness on a hot plate at 125 °C. Partial decomposition of the precursors was observed during drying. The precursor powders were then calcined in air at 525 °C for 2 h to obtain Mn–Ni–Zn ferrite nanopowders.
1 and stirred for 1 h at room temperature using a magnetic stirrer. The pH of the reaction mixture was found to be ∼2. Dark brown precursors were formed when the reaction mixtures were evaporated to dryness on a hot plate at 125 °C. Partial decomposition of the precursors was observed during drying. The precursor powders were then calcined in air at 525 °C for 2 h to obtain Mn–Ni–Zn ferrite nanopowders.
From now onwards, nanocomposites prepared by the one-pot method and physical mixing method will be referred to as ‘composites-OP’ and ‘composites-PM’, respectively.
2.4. Characterization
Room temperature X-ray diffraction spectra of the precursors and the calcined powders were recorded using a powder X-ray diffractometer (Mini Flex II, Rigaku, Japan) with Cu Kα (λ = 0.15405 nm) radiation. Thermogravimetric analysis (TGA) and differential scanning calorimetric (DSC) analysis were carried out on the precursor using a DTG-60 and a DSC-60 (Shimadzu, Japan) respectively in air flow with a heating rate of 10 °C min−1 between 30 to 550 °C. Aluminium and platinum sample pans were used for DSC and TGA respectively. High resolution transmission electron microscope (HRTEM) (JEOL JEM 1400, Japan) images of the samples were employed to analyze the shapes and sizes of the synthesized nanocomposites. Morphology of the nanocomposites prepared by the one-pot method and physical mixing method was studied using scanning electron microscope (SEM) (JSM-6360LV, JEOL, Japan) using an accelerating voltage of 15 kV. Elemental analysis of the composites were performed using energy dispersive X-ray analysis (EDX) which was attached to the SEM. Room temperature magnetization with respect to external magnetic field was measured for the synthesized composites by a vibrating sample magnetometer (EV5, ADE Technology, USA).For measurement of microwave absorptions of the synthesized nanocomposites in the X-band (8.2–12.4 GHz range), HP 8510 vector network analyzer (USA) was used and reflection loss (RL) was calculated using the measured values of complex permittivity and permeability. To prepare the samples for this purpose, nanocomposite powders were first mixed with an aqueous solution of 10 wt% polyvinyl alcohol (PVA) which acted as binder and the mixture was dried. This mixture was further ground to powders and then compressed under a pressure of 10 tons and shaped into rectangular pellets with the size of 10.16 mm × 22.86 mm × 2 mm, so as to fit exactly into a rectangular waveguide of the X-band.
3. Results and discussion
3.1. Thermal analysis
TGA and DSC analysis were used to investigate the thermal decomposition behavior of precursors prepared by the OP method. A total weight loss of ∼95% was observed when the precursor powder was heated from 30 to 550 °C in air (Fig. 1). Initially, about 4% weight loss occurred in the region of 40 to 100 °C due to the loss of moisture from the sample. Then, in the temperature range of 200 to 470 °C, around 91% weight loss was observed. This might be due to the oxidative decomposition of precursor and evolution of CO2 and NOx gases. This decomposition also appeared as an exothermic peak at 429 °C in the DSC thermogram. No weight loss was observed in TGA when the sample was heated beyond 470 °C. This confirmed the full decomposition of carbonaceous mass of the precursor occurred within 470 °C.3.2. XRD analysis
XRD patterns of (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites, prepared by the one-pot method as well as physical mixing method exhibited diffraction peaks corresponding to both spinel Mn0.2Ni0.4Zn0.4Fe2O4 (ref. 40 and 41) and hexagonal BaFe12O19 [ICDD 84-0757] phase (Fig. 2(a) and (b)) and indicated the coexistence of both phases in the composite powders. Any impurity peak, such as NiO, MnO, ZnO, BaO, BaCO3, and α-Fe2O3, was not observed within the resolution of the technique. However, variations in relative intensities of the diffraction peaks were observed for these two types of composites. This might be due to the variation of crystallite size and homogeneous distribution of the spinel and hexagonal phases in the nanocomposites with the method of preparation. The average crystallite sizes of Mn0.2Ni0.4Zn0.4Fe2O4 and BaFe12O19 phases in (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites were calculated by X-ray peak-broadening method using Scherrer's equation42 and listed in Table 2. For Mn0.2Ni0.4 Zn0.4Fe2O4 phase, the diffraction peak at 2θ = 35.6° which corresponds to (311) plane and for BaFe12O19 phase, diffraction peak at 2θ = 34.2°, i.e. (114) plane were used.| Sample | Crystallite size (nm) | |||
|---|---|---|---|---|
| One-pot | Physical mixing | |||
| (114) planea | (311) planeb | (114) planea | (311) planeb | |
| a For BaFe12O19.b For Mn0.2Ni0.4Zn0.4Fe2O4. | ||||
| Mn0.2Ni0.4Zn0.4Fe2O4 | — | 19 | — | — |
| (Mn0.2Ni0.4Zn0.4Fe2O4)0.85–(BaFe12O19)0.15 | 22 | 16 | 43 | 19 |
| (Mn0.2Ni0.4Zn0.4Fe2O4)0.75–(BaFe12O19)0.25 | 27 | 14 | 42 | 17 |
| (Mn0.2Ni0.4Zn0.4Fe2O4)0.5–(BaFe12O19)0.5 | 34 | 12 | 43 | 16 |
| (Mn0.2Ni0.4Zn0.4Fe2O4)0.25–(BaFe12O19)0.75 | 38 | 11 | 45 | 18 |
| BaFe12O19 | 43 | — | — | — |
The important feature was that, in (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites-OP method, the crystallite size of Mn0.2Ni0.4Zn0.4Fe2O4 phase was increased from 11 to 19 nm with an increasing amount of Mn0.2Ni0.4Zn0.4Fe2O4 phase in the composite. The same trend was observed for BaFe12O19 phase and its crystallite size was found to increase from 22 to 43 nm. The crystallite sizes of both spinel and hexagonal phase of (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites-PM method, did not change much with varying amounts of Mn0.2Ni0.4Zn0.4Fe2O4 and BaFe12O19 phases in the composites and were found to be ∼18 nm for Mn0.2Ni0.4Zn0.4Fe2O4 and ∼43 nm for BaFe12O19. These values were almost the same in comparison with the crystallite sizes of pure Mn0.2Ni0.4Zn0.4Fe2O4 and BaFe12O19 nanopowders, indicating that the pure phases retain their individual crystallite sizes in the composites.
3.3. TEM and SEM analysis
Nanocomposites prepared by physical mixing and one-pot method showed two distinct types of microstructures when their morphology was investigated by HRTEM (Fig. 3). In the case of nanocomposites-PM (Fig. 3(d), (f), (h), and (j)), clear segregation of hexagonal BaFe12O19 nanoparticles (average particle size of ∼60–70 nm, Fig. 3(a)) and spherically shaped agglomerated Mn0.2Ni0.4Zn0.4Fe2O4 nanoparticles (∼20 nm average particle size, Fig. 3(b)) were observed. On the contrary, for the nanocomposites prepared by the one-pot method, almost uniformly shaped nanoparticles (average particle size ∼60–70 nm) were observed (Fig. 3(c), (e), (g), and (i)). SEM micrographs of the composites also revealed the intimate coexistence of Mn0.2Ni0.4Zn0.4Fe2O4 and BaFe12O19 particles in the composites-OP (Fig. 4(a)), the presence of large BaFe12O19 and small Mn0.2Ni0.4Zn0.4Fe2O4 nanoparticles in the composites-PM (Fig. 4(b)).Electron microscopic analysis clearly shows that the nanocomposites synthesized by the one-pot method possess better homogeneous mixing of Mn0.2Ni0.4Zn0.4Fe2O4 and BaFe12O19 phases than the composites prepared by physical mixing method. This difference in morphology of the samples may play an important role in the magnetic and microwave absorption properties of the nanocomposites. EDX analysis (Fig. 4(c)) of final nanocomposite indicated the presence of all the elements (e.g., Mn, Ni, Zn, Fe, Ba and O).
Thermal, XRD, and microscopic analyses confirmed that, in one-pot synthesis the calcination of precursor powder leads to the formation of (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites. Here, precursor powders are prepared by mixing aqueous solutions of metal nitrate with EDTA followed by drying of reaction mixture. EDTA, a strong chelating agent, plays a significant role in the formation of nanocomposites. It prevents the segregation or intermittent precipitation of metal ions from solution during evaporation and also helps the formation of a fluffy, voluminous, porous carbon-rich precursor. During decomposition of precursor, nascent metal oxides form small atomic clusters with proper chemical homogeneity, which are embedded into the precursor. These nascent metal oxides when calcined at 800 °C produce desired composite powders.39,43,44
3.4. Magnetic measurements
VSM was used to measure the room temperature magnetization behaviours of (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites prepared by two different methods (OP and PM method) with an applied field of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe and shown in Fig. 5. The most important observation was that composites-OP showed single hysteresis loop, signifying hard and soft phases were well exchange-coupled to each other, whereas composites-PM exhibited a typical two loop “bee waist” type hysteresis loop, indicating the absence of exchange coupling between hard and soft phases.1,24,34,35 Hence, (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites prepared by one-pot method, with crystallographically two phase behaviour demonstrated magnetically good single phase behaviour. Coercivity (Hc) values of the nanocomposites prepared by both methods increased with increasing hard ferrite phase (i.e. BaFe12O19) content in the composite (Table 3).
000 Oe and shown in Fig. 5. The most important observation was that composites-OP showed single hysteresis loop, signifying hard and soft phases were well exchange-coupled to each other, whereas composites-PM exhibited a typical two loop “bee waist” type hysteresis loop, indicating the absence of exchange coupling between hard and soft phases.1,24,34,35 Hence, (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites prepared by one-pot method, with crystallographically two phase behaviour demonstrated magnetically good single phase behaviour. Coercivity (Hc) values of the nanocomposites prepared by both methods increased with increasing hard ferrite phase (i.e. BaFe12O19) content in the composite (Table 3).
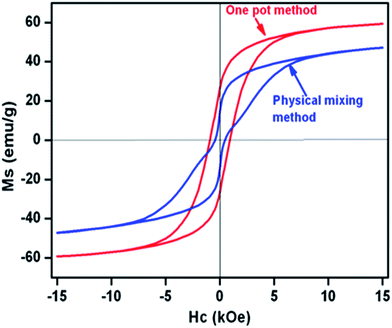 | ||
| Fig. 5 Room temperature hysteresis loops for (Mn0.2Ni0.4Zn0.4Fe2O4)0.85–(BaFe12O19)0.15 nanocomposite prepared by one-pot and physical mixing methods. | ||
| Sample | One-pot | Physical mixing | ||
|---|---|---|---|---|
| Hc (Oe) | Ms (emu g−1) | Hc (Oe) | Ms (emu g−1) | |
| Mn0.2Ni0.4Zn0.4Fe2O4-pure | 61.1 | 39.1 | — | — |
| (Mn0.2Ni0.4Zn0.4Fe2O4)0.85–(BaFe12O19)0.15 | 923.7 | 59.3 | 459.1 | 47.1 |
| (Mn0.2Ni0.4Zn0.4Fe2O4)0.75–(BaFe12O19)0.25 | 1577.7 | 57.6 | 1391.7 | 50.4 |
| (Mn0.2Ni0.4Zn0.4Fe2O4)0.5–(BaFe12O19)0.5 | 3207.3 | 56.9 | 2466.8 | 53.9 |
| (Mn0.2Ni0.4Zn0.4Fe2O4)0.25–(BaFe12O19)0.75 | 4450.9 | 55.8 | 2839.4 | 56.8 |
| BaFe12O19-pure | 4913.9 | 56.5 | — | — |
For composites-OP, the initial incorporation of BaFe12O19 phase caused enhancement of saturation magnetization (Ms) values which was due to the spring exchange coupling between hard and soft magnetic phases. However, subsequent increase of BaFe12O19 content in the (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites (i.e., with increasing (1 − x) values) did not affect Ms value much. Whereas, in the case of composites-PM, Ms values increased with increasing amount of BaFe12O19. Ms and Hc values of all the composites prepared by the one-pot method were higher than those of the composites prepared by the physical mixing method. These facts indicate that hard and soft ferrite phases are sufficiently exchange-coupled to each other in the composites prepared by the one-pot method. According to Moon et al.,36 Ms of hard ferrite and soft ferrite nanocomposites without exchange coupling can be expressed as
| Ms = Ms,h(1 − fs) + Ms,sfs | (1) |
3.5. Microwave absorption study
The complex permittivity and permeability are usually used to analyze the dielectric and magnetic properties of absorber materials. Generally, the real parts (ε′ and μ′) signify the storage capability of dielectric and magnetic energy, whereas the imaginary parts (ε′′ and μ′′) stand for the loss of dielectric and magnetic energy.1,2,8 The reflection loss (RL) was calculated from the complex relative permeability and permittivity at a given frequency and specimen thickness using a model of single-layered plane wave absorber, proposed by Naito and Sutake.47
 | (2) |
 | (3) |
To determine the change of microwave absorption properties with thickness of the absorber, the reflection loss was calculated using eqn (2) and (3) for different absorber thicknesses.
Microwave absorption behavior of (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites with different compositions (x = 0.85, 0.75, 0.5 and 0.25) synthesized by the one-pot method were investigated. Loss tangent was plotted against frequency in order to understand the particular loss mechanism for each composite and is shown in Fig. 6(a) and (b). The dielectric and magnetic loss tangents can be expressed as tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δε = ε′′/ε′ and tan
δε = ε′′/ε′ and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δμ = μ′′/μ′, respectively. Fig. 6(b) shows that these composites experience only magnetic loss parameter whereas dielectric loss parameter is negligible (Fig. 6(a)).
δμ = μ′′/μ′, respectively. Fig. 6(b) shows that these composites experience only magnetic loss parameter whereas dielectric loss parameter is negligible (Fig. 6(a)).
Reflection loss was calculated using eqn (2) and (3) and plotted against frequency for different (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites (x = 0.85, 0.75, 0.5 and 0.25) synthesized by the OP method for a thickness of 3 mm (Fig. 6(c)). In the plot of reflection loss vs. frequency, the dip of the curves were designated for maximum absorption i.e., minimum reflection loss. It was observed that, with the decreasing value of x (i.e., increasing BaFe12O19 content in the composite), maximum absorption would decrease. As the composite having composition (Mn0.2Ni0.4Zn0.4Fe2O4)0.85–(BaFe12O19)0.15, exhibited minimum reflection loss (∼−15 dB), i.e., maximum absorption compared to other composites, so this composition was chosen for further studies and its microwave absorption properties were compared with the composite prepared by PM method as well as pure Mn0.2Ni0.4Zn0.4Fe2O4 and BaFe12O19.
The real and imaginary permittivity (Fig. 7(a) and (b)) and permeability (Fig. 7(c) and (d)) for the nanocomposites synthesized by both methods, pure BaFe12O19 and Mn0.2Ni0.4Zn0.4Fe2O4 nanopowders, were plotted as a function of frequency in the X-band range (8.2–12.4 GHz). It was observed that over the entire frequency range, ε′ values remained almost constant. The ε′ values for both nanocomposites were in between the values of pure BaFe12O19 and Mn0.2Ni0.4Zn0.4Fe2O4 (Fig. 7(a)). The imaginary dielectric parameter (ε′′) values remained almost constant except for a broad peak in the range of 9.88 GHz to 12.4 GHz for the composite-OP (Fig. 7(b)). The intrinsic, electric dipole polarization and interfacial polarization are mainly responsible for this kind of behavior.4 The real permeability (μ′) values of composite and pure nanopowders remained almost constant throughout the entire frequency range (Fig. 7(c)). The imaginary permeability (μ′′) values (Fig. 7(d)) showed a decreasing tendency from 8.2 GHz to 12.4 GHz for both the nanocomposites and pure ferrites; however, maximum imaginary permeability was observed for the composite synthesized by the one-pot method.
Refection loss for nanocomposites as well as pure ferrite nanopowders was calculated for the absorber thickness of 3 mm (Fig. 8(a)). The estimated electromagnetic wave absorption values are listed in Table 4. In Fig. 8(a), it was observed that, composite-OP method showed greater reflection loss (∼−15 dB at 9.88 GHz corresponds to ∼96.84% absorption) than composite-PM method (∼−8.7 dB at 8.62 GHz). Pure hard and soft ferrite nanopowders showed reflection losses lower than −10 dB (∼−7 dB and ∼−6 dB respectively). Composite-OP method showed >10 dB reflection loss (i.e., >90% absorption) over the frequency range of 8.2 GHz to 10.6 GHz. Fig. 8(b) illustrates the reflection loss vs. frequency for one-pot synthesized nanocomposites with different thicknesses of the absorber.
| Sample | Minimum RL (dB) | Frequency (GHz) |
|---|---|---|
| BaFe12O19-pure | −5.8 | 8.62 |
| (Mn0.2Ni0.4Zn0.4Fe2O4)0.85–(BaFe12O19)0.15 (one-pot method) | −15 | 9.88 |
| (Mn0.2Ni0.4Zn0.4Fe2O4)0.85–(BaFe12O19)0.15 (physical mixing method) | −8.7 | 8.62 |
| Mn0.2Ni0.65Zn0.35Fe2O4-pure | −7.6 | 10.3 |
The reflection loss was found to increase with increasing thickness of the sample until 3.5 mm with the shifting of the frequency corresponding to the maximum loss towards lower frequency.
Reflection loss of ∼−25 dB (i.e., >99% absorption) was observed at 8.2 GHz for an absorber thickness of 3.5 mm but frequency range corresponding to reflection loss >−10 dB (means >90% absorption) becomes narrower than the specimen thickness of 3 mm. The reflection loss value of −20 dB is equivalent to 99% absorption, which is considered satisfactory for microwave absorption.
The improved microwave absorption property of hard–soft ferrite nanocomposites has already been reported by Shen et al.,1 Tyagi et al.,2 Chen et al.,26 and Xie et al.,38 and this enhancement of microwave absorption property was attributed to the exchange spin coupling interaction between hard and soft ferrite phases. Hence, in the present case the observed enhancement of microwave absorbing properties of the composites, prepared by the one-pot method, was also considered due to the exchange coupling interaction between the hard (BaFe12O19) and soft (Mn0.2Ni0.4Zn0.4Fe2O4) ferrite phases.
In the composites (OP method), hard ferrite phase and soft ferrite phase are coupled to each other by an exchange through interfacial interaction which influences the relative complex permeability of the materials.2 Interfacial interaction between the two phases is one of the important factors for the microwave absorption in the GHz frequency range.1
The interfacial multipoles in nanocomposite cause the surface spin of ferrite nanoparticles to become disordered, which leads to high magnetic loss. Therefore, microwave absorption improves.1 There will be stronger exchange coupling interactions at the interface if the grain size is smaller.2,26 In addition, the nanocomposites prepared by OP method are made of nanoparticles and both Mn0.2Ni0.4Zn0.4Fe2O4 and BaFe12O19 nanoparticles are homogeneously mixed, which have a small size effect and enhanced spring exchange coupling interaction. In a hard–soft ferrite system, three types of magnetic interactions are present. The most important one is the exchange-coupling interaction between soft and hard phases, while the remaining ones are dipolar interactions between the hard–hard and soft–soft ferrite phases.1,48
The hard ferrite possesses a high magnetocrystalline anisotropic energy compared to soft ferrite. If the hard ferrite grains are sufficiently exchange-coupled with the neighbouring soft ferrite grains, then the exchange-coupled interaction will not only help to align the magnetization in the soft ferrite phases but also helps to arrange magnetic moments of the hard and soft ferrite phases parallel to each other in hard soft ferrite nanocomposites. This leads to higher energy product and enhances the reflection loss in microwave absorption.1,27
In the one-pot synthesis method, as both the phases are grown together from a single reaction mixture, hence intimate co-existence of nanosized hard and soft ferrite phases were observed in these nanocomposites (Fig. 3(c), (e), (g) and (i)). This fact leads to sufficient exchange coupling between hard and soft ferrite phases, and supply marvellous opportunities for absorbing microwave and dissipating energy.
4. Conclusion
A novel ‘one-pot’ synthetic methodology has been reported for the synthesis of (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites. In this method, as hard ferrite phase (BaFe12O19) and soft ferrite phase (Mn0.2Ni0.4Zn0.4Fe2O4) both were grown together from a single reaction mixture, their intimate mixing in the final composite was observed. In the nanocomposites prepared by the one-pot method, there was better homogeneous distribution of the hard and soft phases than in the nanocomposites prepared by the physical mixing method. Hard–soft ferrite nanocomposites thus formed (OP method) possessed excellent exchange spring coupling behaviour with high saturation magnetization as well as high coercivity. These composites also exhibited very good microwave absorption property in the X-band region. Nanocomposites prepared by one-pot method demonstrated superior magnetic as well as microwave absorption properties in comparison with the nanocomposites prepared by physical mixing method and pure BaFe12O19 and Mn0.2Ni0.4Zn0.4Fe2O4 nanoparticles.This one-pot method offers several advantages such as (i) preparation method is simple, (ii) water is used as solvent, (iii) cheap metal nitrates are used as starting materials, (iv) unlike hydrothermal or sol–gel method, this method can be operated in open atmosphere and complicated reaction set up is not required, (v) composition of nanocomposites can simply be manipulated by mixing appropriate amount of metal nitrate solutions. The novelty of this method lies in its simplicity, the requirement of relatively low calcination temperatures and cost-effectiveness. Simple method of preparation, and superior magnetic and microwave absorption properties make these nanocomposites potential candidates for application in next generation permanent magnets as well as radar absorbing materials.
The one-pot method has also shown its capability of producing nanocomposites with a variety of compositions. Employing this one-pot method several other hard–soft ferrites nanocomposites, such as NixZn(1−x)Fe2O4–BaFe12O19, NixZn(1−x)Fe2O4–SrFe12O19, NiFe2O4–SrFe12O19, and Mn0.2NixZn(1−x)Fe2O4–SrFe12O19, have also been prepared and results will be communicated shortly.
Acknowledgements
N. N. Ghosh gratefully acknowledges financial support from DRDO, New Delhi India (ERIP/ER/1305004/M/01/1523). S. Hazra thanks CSIR, India, for a Senior Research Fellowship. We also express our thanks to Dr Rahul Mohan, NCAOR, Goa, India, Prof. Paul A. Millner and Mr Martin Fuller, University of Leeds, U K, for recording SEM and TEM micrographs.Notes and references
- X. Shen, F. Song, J. Xiang, M. Liu, Y. Zhu and Y. Wang, J. Am. Ceram. Soc., 2012, 95, 3863 CrossRef CAS PubMed.
- S. Tyagi, H. B. Baskey, R. C. Agarwala, V. Agarwala and T. C. Shami, Ceram. Int., 2011, 37, 2631 CrossRef CAS PubMed.
- L. G. Yan, J. B. Wang, X. H. Han, Y. Ren, Q. F. Liu and F. S. Li, Nanotechnology, 2010, 21, 095708 CrossRef PubMed.
- R. C. Pullar, J. D. Breeze and N. M. Alford, J. Am. Ceram. Soc., 2005, 88, 2466 CrossRef CAS PubMed.
- X. H. Guo, Y. H. Deng, D. Gu, R. C. Che and D. Y. Zhao, J. Mater. Chem., 2009, 19, 6706 RSC.
- X. L. Dong, X. F. Zhang, H. Huang and F. Zuo, Appl. Phys. Lett., 2008, 92, 013127 CrossRef PubMed.
- W. L. Song, M. S. Cao, Z. L. Hou, J. Yuan and X. Y. Fang, Scr. Mater., 2009, 61, 201 CrossRef CAS PubMed.
- P. Bhattacharya, S. Dhibar and C. K. Das, Polym.-Plast. Technol. Eng., 2013, 52, 892 CrossRef CAS.
- Q. Ling, J. Sun, Q. Zhao and Q. Zhou, Polym.-Plast. Technol. Eng., 2010, 49, 481 CrossRef CAS.
- V. Gupta, M. K. Patra, A. Shukla, L. Saini, S. Songara, R. Jani, S. R. Vadera and N. Kumar, J. Nanopart. Res., 2012, 14, 1271 CrossRef.
- P. Bhattacharya, S. Dhibar, G. Hatui, A. Mandal, T. Das and C. K. Das, RSC Adv., 2014, 4, 17039 RSC.
- M. Zong, Y. Huang, Y. Zhao, X. Sun, C. Qu, D. Luo and J. Zheng, RSC Adv., 2013, 3, 23638 RSC.
- Y. Sun, F. Xiao, X. Liu, C. Feng and C. Jin, RSC Adv., 2013, 3, 22554 RSC.
- S. Hazra and N. N. Ghosh, J. Nanosci. Nanotechnol., 2014, 14, 1983 CrossRef CAS PubMed.
- P. Pant, S. Bhuvaneswari and N. N. Ghosh, Recent Pat. Nanotechnol., 2008, 2, 8 CrossRef CAS.
- R. S. Meena, S. Bhattachrya and R. Chatterjee, Mater. Des., 2010, 31, 3220 CrossRef CAS PubMed.
- R. S. Meena, S. Bhattachrya and R. Chatterjee, Mater. Sci. Eng., B, 2010, 171, 133 CrossRef CAS PubMed.
- R. S. Meena, S. Bhattachrya and R. Chatterjee, J. Magn. Magn. Mater., 2010, 322, 1923 CrossRef CAS PubMed.
- R. Ji, C. Cao, Z. Chen, H. Zhai and J. Bai, J. Mater. Chem. C, 2014, 2, 5944 RSC.
- A. Ghasemi, A. Hossienpour, A. Morisako, A. Saatchi and M. Salehi, J. Magn. Magn. Mater., 2006, 302, 429 CrossRef CAS PubMed.
- A. Ghasemi, A. Hossienpour, A. Morisako, X. X. Liu and A. Ashrafizadeh, Mater. Des., 2008, 29, 112 CrossRef CAS PubMed.
- M. Sugimoto, J. Am. Ceram. Soc., 1999, 82, 269 CrossRef CAS PubMed.
- E. F. Kneller and R. Hawig, IEEE Trans. Magn., 1991, 27, 3588 CrossRef CAS.
- F. Song, X. Shen, M. Liu and J. Xiang, J. Colloid Interface Sci., 2011, 354, 413 CrossRef CAS PubMed.
- J. R. Liu, M. Itoh and K. Machida, Appl. Phys. Lett., 2006, 88, 062503 CrossRef PubMed.
- N. Chen, G. Mu, X. Pan, K. Gan and M. Gu, Mater. Sci. Eng., B, 2007, 139, 256 CrossRef CAS PubMed.
- T. Maeda, S. Sugimoto, T. Kagotani, N. Tezuka and K. Inomata, J. Magn. Magn. Mater., 2004, 281, 195 CrossRef CAS PubMed.
- D. Goll, M. Seeger and H. Kronmuller, J. Magn. Magn. Mater., 1998, 185, 49 CrossRef CAS.
- H. W. Kwon, I. C. Jeong, A. S. Kim, D. H. Kim, S. Namkung, T. S. Jang and D. H. Lee, J. Magn. Magn. Mater., 2006, 304, e219 CrossRef CAS PubMed.
- J. S. Jiang, J. E. Pearson, Z. Y. Liu, B. Kabius, S. Trasobares, D. J. Miller, S. D. Bader, D. R. Lee, D. Haskel, G. Srajer and J. P. Liu, J. Appl. Phys., 2005, 97, 10K311 Search PubMed.
- J. P. Liu, Y. Liu, R. Skomaski and D. J. Sellmyer, J. Appl. Phys., 1999, 85, 4812 CrossRef CAS PubMed.
- C. Sudakar, G. N. Subbanna and T. R. N. Kutty, J. Appl. Phys., 2003, 94, 6030 CrossRef CAS PubMed.
- M. Pal, S. Bid, S. K. Pradhan, B. K. Nath, D. Das and D. Chakravorty, J. Magn. Magn. Mater., 2004, 269, 42 CrossRef CAS.
- D. Roy and P. S. A. Kumar, J. Appl. Phys., 2009, 106, 073902 CrossRef PubMed.
- D. Roy, C. Shivakumara and P. S. A. Kumar, J. Magn. Magn. Mater., 2009, 321, L11 CrossRef CAS PubMed.
- K. W. Moon, S. G. Cho, Y. H. Choa, K. H. Kim and J. Kim, Phys. Status Solidi A, 2007, 204, 4141 CrossRef CAS.
- S. Hazra, M. K. Patra, S. R. Vadera and N. N. Ghosh, J. Am. Ceram. Soc., 2012, 95, 60 CrossRef CAS PubMed.
- T. Xie, L. Xu and C. Liu, RSC Adv., 2013, 3, 15856 RSC.
- A. Rajput, S. Hazra, G. F. Fernando and N. N. Ghosh, Synth. React. Inorg. Met.-Org. Chem., 2011, 41, 1114 CrossRef CAS.
- S. Hazra, M. K Patra, S. R. Vadera and N. N. Ghosh, Optoelectron. Adv. Mater., Rapid Commun., 2012, 6, 451 CAS.
- A. Verma and R. Chatterjee, J. Magn. Magn. Mater., 2006, 306, 313 CrossRef CAS PubMed.
- B. D. Cullity, in Elements of X-ray Diffraction, Addison-Wesley, New York, 1978, p. 99 Search PubMed.
- R. N. Das, A. Pathak and P. Pramanik, J. Am. Ceram. Soc., 1998, 81, 3357 CrossRef CAS PubMed.
- P. P. Sarangi, B. Naik and N. N. Ghosh, J. Am. Ceram. Soc., 2008, 91, 4145 CrossRef CAS PubMed.
- S. M. A. Radmanesh and S. A. S. Ebrahimi, J. Supercond. Novel Magn., 2013, 26, 2411 CrossRef CAS PubMed.
- A. Xia, S. Ren, C. Zuo, L. Zhang, M. Xie, Y. Deng, R. Wu, W. Xu, C. Jin and X. Liu, RSC Adv., 2014, 4, 18885 RSC.
- N. Yoshiyuoi and S. Kunihiro, IEEE Trans. Microwave Theory Tech., 1971, 19, 65 CrossRef.
- R. W. Gao, W. C. Feng, H. Q. Liu, B. Wang, W. Chen and G. B. Han, J. Appl. Phys., 2003, 94, 664 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Room temperature M–H curve for (Mn0.2Ni0.4Zn0.4Fe2O4)x–(BaFe12O19)1−x nanocomposites (x = 0.85, 0.75, 0.5 and 0.25) prepared by one-pot and physical mixing method. See DOI: 10.1039/c4ra07145j |
| This journal is © The Royal Society of Chemistry 2014 |







