One-step fabrication of a superhydrophobic polymer surface from an acrylic copolymer containing POSS by spraying†
Abstract
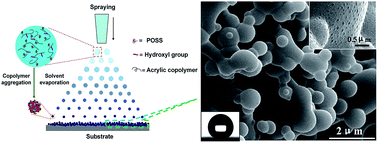
A superhydrophobic polymer surface with hierarchical structures is prepared by a one-step spraying process with a POSS-acrylic random copolymer solution. The morphology of the superhydrophobic surface consists of micron/nano-scale spherical protrusions. The possible formation mechanism of the superhydrophobic surface is proposed. The as-prepared surface has good superhydrophobicity and blood compatibility.

 Please wait while we load your content...
Please wait while we load your content...