Preparation and characterization of continuous carbon nanofiber-supported SPEEK composite membranes for fuel cell application
Abstract
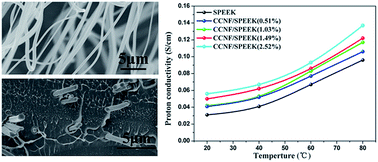
An easy spinning-based strategy was developed to fabricate polyacrylonitrile nanofibers as a precursor to obtaining continuous carbon nanofiber (CCNF) mats after carbonization. The composite membrane was then prepared by incorporating CCNFs into sulfonated poly(ether ether ketone) (SPEEK) for application in proton-exchange membrane fuel cells. Scanning electron microscopy, X-ray diffraction, thermogravimetric analysis, and tensile strength tests were used to characterize plain SPEEK and composite membranes. All dense composite membranes were found to have excellent water swelling, high mechanical performance, good proton conductivity, and low methanol permeability. The composite membrane with 0.51 wt% CCNFs displayed a proton conductivity of 0.041 S cm−1 at room temperature and was fully hydrated. Moreover, the relative selectivity of the hybrid membrane with 2.52 wt% CCNFs was 1.5 times higher than that of a pure SPEEK membrane. These results showed that the CCNF-supported SPEEK membranes were promising polyelectrolyte membranes for fuel cell applications.

 Please wait while we load your content...
Please wait while we load your content...