A novel supported Cu catalyst with highly dispersed copper nanoparticles and its remarkable catalytic performance in methanol decomposition†
Shaojun Qing‡
a,
Xiaoning Hou‡a,
Yajie Liua,
Hongjuan Xia,
Xiang Wangb,
Cheng-meng Chenc,
Zhiwei Wud and
Zhixian Gao*a
aInstitute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China. E-mail: gaozx@sxicc.ac.cn
bInstitute of Applied Chemistry, Department of Chemistry, Nanchang University, Nanchang 330031, China
cKey Laboratory of Carbon Materials, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China
dState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China
First published on 8th October 2014
Abstract
Using starch modified SiO2 as the support, an efficient copper catalyst with superior catalytic performance for methanol decomposition can be obtained, suggesting the key role of the nature of the support in preparing Cu/SiO2 catalysts with stable and highly dispersed copper nanoparticles using an impregnation method.
Because of their low cost and good catalytic activity, Cu/SiO2 catalysts have been widely investigated in many catalytic reactions, such as hydrogenolysis of esters to alcohols,1 dehydrogenation of alcohols,2 decomposition of methanol,3 etc. However, deactivation of Cu/SiO2 is unavoidable during the reaction process. This can be attributed to the weak interaction between copper and SiO2,4 and to the inferior thermostability of copper due to its low Hüttig temperature,5 so copper metal agglomerates and sinters easily, which shows a gradual decrease of activity with time on stream.
Therefore, many research efforts toward stable Cu catalysts have been carried out aiming at enhancing the stability of Cu/SiO2 catalyst. The improvement has been achieved by using novel preparation methods such as atomic layer epitaxy technique,6 electroless,7 deposition precipitation,8 ion-exchange9 and ammonia evaporation hydrothermal,10 and by modification with a second element.11 These studies clearly demonstrate that higher dispersion of copper helps delaying the agglomeration of copper, giving a catalyst with enhanced catalytic performance. The dispersion of copper is greatly affected by the preparation method, and the catalyst prepared via the impregnation method exhibit poor copper dispersion and inferior catalytic activity.6,7,9a,9b
The preparations of Cu supported on SiO2 by impregnation method have been widely adopted due to its simplicity and the easy control of conditions. However, good results have been obtained only with M-Cu/SiO2 systems. For example, Akio TADA12 prepared a catalyst with high selectivity and stability for methanol decomposition by using nickel to modify Cu/SiO2. Ching-Shiun Chen13 reported that the dispersion of copper could be significantly improved by modification with Fe, and both the activity and stability were enhanced for the reverse water gas shift reaction. Up till now, little work has been done on enhancing catalytic performance of Cu/SiO2 via modifying the SiO2 support. Our previous work14 showed that using the starch modified SiO2 as support an efficient Cu/SiO2 for preparing methyl formate by dehydrogenation of methanol at 200 °C could be obtained by impregnation method, and the results were attributed to the surface silanol (Si–OH) concentration being modified by starch, which were beneficial to the dispersion of Cu species. In this contribution, the catalyst system is further researched aiming at a better understanding of the mechanism of starch modification and the catalytic performance for methanol decomposition at 300 °C.
The starch modified SiO2 support (denoted as SiO2-S) calcinated at 500 °C was denoted as SiO2-S-500 (starch was completely removed from the support, Fig. S1, ESI†). After loading 22.5% of Cu via impregnation, the samples were subjected to pre-calcination in different atmosphere at 300 °C, giving four catalysts for catalytic testing. (Details are listed in ESI†).
Fig. 1 presented catalytic performance of the above four catalysts for methanol decomposition, and among which Cu/SiO2-S[300-Air] exhibit the best activity and stability. The initial methanol conversion was 91.8% and decreased to 64.0% at 30 h, then levelled off up to 170 h. However, on Cu/SiO2[300-Air] catalyst, the initial methanol conversion was only 45.7%, and dropped drastically to 16.9% within 4.4 h. The data clearly indicated that the catalytic behaviour of Cu/SiO2 catalyst was significantly enhanced due to the pre-modification of support by starch prior to Cu loading.
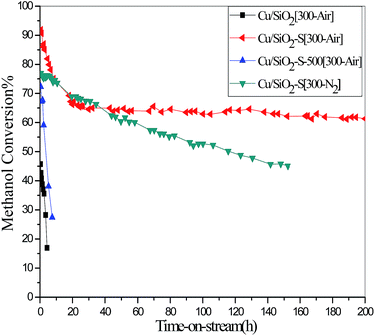 | ||
| Fig. 1 The variations of methanol conversion with time on stream. Reaction conditions: temperature = 300 °C, Weight Hourly Space Velocity (WHSV) = 1.58 h−1, pressure = 0.1 MPa. | ||
The evaluation data of Cu/SiO2-S[300-N2] showed an initial methanol conversion of 77.0%, then gradually dropped to 44.9% within 153 h, with no stable platform appeared. In a control experimental, when the support was pre-calcinated in air to remove the starch, the obtained Cu/SiO2-S-500[300-Air] revealed very rapid deterioration in catalytic activity, which is similar to that of bare Cu/SiO2[300-Air]. These data demonstrated that the effect of starch on improving the catalytic performance occurred during the process of catalyst preparation and correlated well with the calcination atmosphere.
On the four catalysts, the major reaction of methanol was decomposition, and the selectivity of H2 and CO was more than 96%.
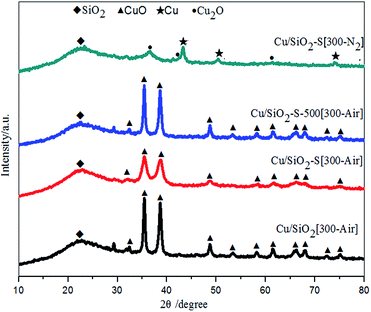
To understand the difference in catalytic performance, the fresh catalysts (after calcination) were characterized. With XRD (Fig. 2), the typical lines assigned to CuO (JCPDS no. 45-1548) were recorded except Cu/SiO2-S[300-N2]. Cu/SiO2-S[300-Air] displayed broadened and decreased diffraction peaks as compared with both Cu/SiO2[300-Air] and Cu/SiO2-S-500[300-Air]. This indicated that CuO particles were smaller (Table S1 ESI†) with a higher dispersion in Cu/SiO2-S[300-Air], comparing with the other two catalysts.11b This was also demonstrated by the results of H2-TPR (Fig. S2, ESI†), XPS (Fig. S3 and Table S1, ESI†) and TEM-HRTEM (Fig. S4, ESI†).
The Cu/SiO2[300-Air] and Cu/SiO2-S[300-Air] were chosen to be pre-reduced at 300 °C by H2 for two hours, and then characterized by TEM and HRTEM (Fig. 3). In the HRTEM image, Cu(111) planes with d-spacing of 0.208 nm was identified, indicating the formation of metal Cu after reduction.15 Obviously, Cu particles in the Cu/SiO2[300-Air] sintered seriously (Fig. 3A), while the Cu agglomeration in the Cu/SiO2-S[300-Air] was hindered, producing smaller Cu particles, less than 8 nm, and highly dispersed on the support (Fig. 3C). The above results were further supported by characterizations with XRD (Fig. S5, ESI†) and dissociative N2O adsorption (Table S1, ESI†). The used catalysts displayed similar XRD patterns (Fig. S5, ESI†) to those of reduced ones, but with increased intensities, indicating agglomeration of Cu metal during the reaction process. Considering the testing duration was 200 h for Cu/SiO2-S[300-Air], while only 4.4 h for Cu/SiO2[300-Air], it could be inferred that the agglomeration rate of Cu was much slower for the former. The XRD patterns of both reduced and used catalysts (Fig. S5, ESI†) revealed a small portion of Cu2O phase, suggesting air oxidation of Cu at room temperature.16
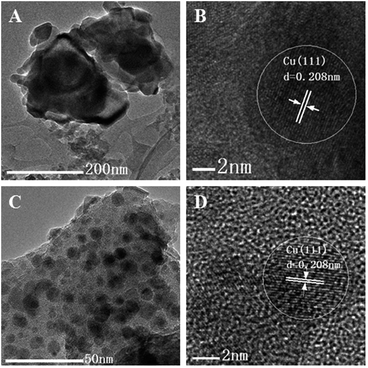 | ||
| Fig. 3 TEM and HRTEM images of reduced catalysts. A and B: Cu/SiO2[300-Air], C and D: Cu/SiO2-S[300-Air]. | ||
On the basis of above results, it was concluded that the high activity and good stability of Cu/SiO2-S[300-Air] catalyst correlate well with the highly dispersed small and stable copper metal particles, which results from the using of starch modified SiO2 as the support.
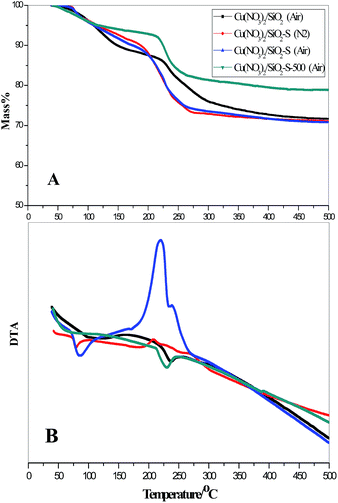
In order to discuss the mechanism of starch modification, the precursors of catalysts (before calcination) were characterized by TG-DTA (Fig. 4). The rate of weight loss was faster for Cu(NO3)2/SiO2-S than that of Cu(NO3)2/SiO2 and Cu(NO3)2/SiO2-S-500 (Fig. 4A). Simultaneously, the process was observed to be exothermic for Cu(NO3)2/SiO2-S, while endothermic for both Cu(NO3)2/SiO2 and Cu(NO3)2/SiO2-S-500 (Fig. 4B). In the N2 atmosphere, the heat release with Cu(NO3)2/SiO2-S was ascribed to the redox reaction between the starch and Cu(NO3)2, which was confirmed by formation of Cu as identified by XRD (Fig. 2). When air was used as the calcination atmosphere, the heat release was stronger, which was attributed to the additional starch oxidation by air. Consequently, decomposition of Cu(NO3)2 started at lower temperatures with an accelerated rate, thus resulting in the formation of highly dispersed CuO on the support.
In conclusion, using starch modified SiO2 as the support, an efficient supported copper based catalyst has been obtained. Characterization data demonstrate that highly dispersed copper species, CuO or Cu, exist as small particles, and their agglomeration and sintering is restrained, thus revealing high activity and stability during the catalytic reaction. The suggested mechanism of starch modification is that the Cu(NO3)2 decomposition process is facilitated by the presence of starch in the support, and further study is going on in the author's lab. The results strongly indicate that the property of support plays a crucial role in preparing an efficient catalyst, which pave a way for stabilizing usually unstable metal species such as copper by pre-modification of the support.
Acknowledgements
The authors thank the financial support from the Innovation Foundation of Institute of Coal Chemistry, Chinese Academy of Sciences (2011SQNRC03).Notes and references
- (a) C. Wen, Y. Y. Cui, W. L. Dai, S. H. Xie and K. N. Fan, Chem. Commun., 2013, 49, 5195 RSC; (b) Z. He, H. Q. Lin, P. He and Y. Z. Yuan, J. Catal., 2011, 277, 54 CrossRef CAS PubMed; (c) F. T. van de Scheur, B. van deir Linden, M. C. Mittelmeijer-Hazeleger, J. G. Nazloomian and L. H. Staat, Appl. Catal., A, 1994, 111, 63 CrossRef CAS; (d) D. M. Montl, M. S. Wal-nwrlght and D. L. Trlmm, Ind. Eng. Chem. Prod. Res. Dev., 1985, 24, 297 CrossRef.
- (a) T. Sodesawa, M. Nagacho, A. Onodera and F. Nozaki, J. Catal., 1986, 102, 460 CrossRef CAS; (b) A. J. Marchi, J. L. G. Fierro, J. Santamaria and A. Monzon, Appl. Catal., A, 1996, 142, 375 CrossRef CAS.
- (a) Ian A. Fisher and A. T. Bell, J. Catal., 1999, 184, 357 CrossRef CAS; (b) D. B. Clarke, D. K. Lee, M. J. Sandoval and A. T. Bell, J. Catal., 1994, 150, 81 CrossRef CAS; (c) S. D. Jackson, D. S. Anderson, G. J. Kelly, T. Lear, D. Lennon and S. R. Watson, Top. Catal., 2003, 22, 3 CrossRef.
- (a) J. E. E. Baglin, Nucl. Instrum. Methods Phys. Res., Sect. B, 1992, 65, 119 CrossRef; (b) J. B. Zhou, H. C. Lu, T. Gustafsson and E. Garfunkel, Surf. Sci., 1993, 293, L887 CrossRef CAS; (c) L. C. A. van den Oetelaar, A. Partridge, S. L. G. Toussaint, C. F. J. Flipse and H. H. Brongersma, J. Phys. Chem. B, 1998, 102, 9541 CrossRef CAS.
- (a) M. S. Spencer, Nature, 1985, 323, 685 CrossRef; (b) M. V. Twigg and M. S. Spencer, Appl. Catal., A, 2001, 212, 161 CrossRef CAS; (c) X. Zhang, B. W. Wang, Y. Y. Guo and G. H. Xu, J. Fuel Chem. Technol., 2011, 39, 702 CAS.
- C. S. Chen, J. H. Lin and T. W. Lai, Chem. Commun., 2008, 40, 4983 RSC.
- C. Y. Shiau and J. C. Tsai, J. Chem. Technol. Biotechnol., 1998, 73, 414 CrossRef CAS.
- E. G. M. Kuijpers, R. B. Tjepkema, W. J. J. van der Wal, C. M. A. M. Mesters, S. F. G. M. Spronck and J. W. Geus, Appl. Catal., 1986, 25, 139 CrossRef CAS.
- (a) T. Sodesawa, React. Kinet. Catal. Lett., 1984, 24, 259 CrossRef CAS; (b) W. C. Zhu, L. X. Wang, S. Y. Liu and Z. L. Wang, React. Kinet. Catal. Lett., 2008, 93, 93 CrossRef CAS; (c) J. C. Lee, D. L. Trimm, M. A. Kohler, M. S. Wainwright and N. W. Cant, Catal. Today, 1988, 2, 643 CrossRef CAS.
- J. L. Gong, H. R. Yue, Y. J. Zhao, S. Zhao, L. Zhao, J. Lv, S. P. Wang and X. B. Ma, J. Am. Chem. Soc., 2012, 134, 13922 CrossRef CAS PubMed.
- (a) S. Zhao, H. R. Yue, Y. J. Zhao, B. Wang, Y. C. Geng, J. Lv, S. P. Wang, J. L. Gong and X. B. Ma, J. Catal., 2013, 297, 142 CrossRef CAS PubMed; (b) S. H. Zhu, X. Q. Gao, Y. L. Zhu, Y. F. Zhu, H. Y. Zheng and Y. W. Li, J. Catal., 2013, 303, 70 CrossRef CAS PubMed.
- A. Tada, Y. Watarai, K. Takahashi, Y. Imizu and H. Itoh, Chem. Lett., 1989, 543 CrossRef CAS.
- C. S. Chen, W. H. Cheng and S. S. Lin, Chem. Commun., 2001, 1770 RSC.
- C. T. Gu, G. J. Li, Y. Q. Hu, S. J. Qing, X. N. Hou and Z. X. Gao, J. Fuel Chem. Technol., 2012, 40, 1328 CrossRef CAS.
- (a) H. Bahmanpour, K. M. Youssef, J. Horky, D. Setman, M. A. Atwater, M. J. Zehetbauer, R. O. Scattergood and C. C. Koch, Acta Mater., 2012, 60, 3340 CrossRef CAS PubMed; (b) K. Pan, H. Ming, H. Yu, H. Huang, Y. Liu and Z. H. Kang, Dalton Trans., 2012, 41, 2564 RSC.
- (a) H. J. Xi, G. J. Li, S. J. Qing, X. N. Hou, J. Z. Zhao, Y. J. Liu and Z. X. Gao, J. Fuel Chem. Technol., 2013, 41, 998 CAS; (b) Y. W. Yang, Z. H. Lu, Y. J. Hu, Z. J. Zhang, W. M. Shi, X. S. Chen and T. T. Wang, RSC Adv., 2014, 4, 13749–13752 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: More experimental details and results of catalyst characterization. See DOI: 10.1039/c4ra10101d |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2014 |