Applications of mesoporous titanium phosphonate functionalized with carboxylic groups
Abstract
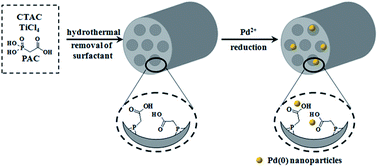
Mesoporous titanium phosphonate functionalized with –COOH was synthesized by a simple co-condensation process with the use of 2-phosphonoacetic acid and titanium tetrachloride under acid conditions. The obtained mesoporous materials show high surface area and large pore volume, which were supported by XRD, TEM, SEM and N2 adsorption analysis. The integrity of the organophosphonate groups were further characterized by FTIR and NMR. Palladium nanoparticles were successfully supported onto the materials combined with a metal adsorption–reduction procedure, showing high activity for the Suzuki reaction. Furthermore, the obtained catalyst can be recovered and reused without significant decrease in catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...