Optimization of one-step and one-substrate synthesis of carbon nanodots by microwave pyrolysis†
Effat Kianpour and
Saeid Azizian*
Department of Physical Chemistry, Faculty of Chemistry, Bu-Ali Sina University, Hamedan 65167, Iran. E-mail: sazizian@basu.ac.ir; sdazizian@yahoo.com; Fax: +98 811 8380709; Tel: +98 811 8282807
First published on 26th August 2014
Abstract
The effect of water on the optical properties and concentration of carbon nanodots (CND), prepared by microwave heating of a mixture of polyethylene glycol 200 (PEG-200) and water, was studied. It was shown that the presence of water as a substrate leads to the synthesis of CND with lower yield and optical properties and it is better to use only PEG-200 as the substrate. Microwave power and irradiation time as two important variables in the synthesis of CND from a single substrate (PEG-200) were optimized to obtain CND with a high yield. The CND obtained under the best conditions were characterized by TEM, XRD, UV-Visible, fluorescence spectroscopy and fluorescence imaging. Consequently, photostability of the prepared CND in acidic and alkaline mediums was studied. Finally, it was shown that the produced CND can be used as a green and cheap reagent for fast detection of metronidazole based on fluorescence quenching.
Fluorescent semiconductor quantum dots (QD) have gained more attention due to potential applications in biological labelling, bioimaging, optoelectronics, analytical chemistry and so on.1,2 But these materials are generally made up of heavy metals which are expensive and environmentally hazardous.3,4 Recently, fluorescent carbon nanodots (CND), with smaller size than QD, have become a promising alternative for QD due to a variety of properties such as stable photoluminescent (PL), low toxicity, good biocompatibility and environmentally-friendly.3,5,6 The synthesis methods of CND have been classified in two main strategies: top-down and bottom-up, most of which require complex, expensive, energy- and time-consuming conditions.3,7–9 Among them microwave assisted pyrolysis, a bottom-up approach, is fast, low-cost, energy efficient and facile technique for production of CND and has attracted much interest in the past few years.3,4,7,8,10–12 Up to now, different carbon nanodots with varying properties have been synthesized. More recently, Zhu et al.3 reported a simple and clean method for synthesis of CND via microwave pyrolysis of saccharide as a carbon source and polyethylene glycol-200 (a non-toxic and non-immunogenic polymer approved by Food and Drug Administration (FDA), USA13) as the passivation agent. Then Amit and coworker demonstrated that polyethylene glycol-200 (PEG-200), dissolved in water, plays dual roles as carbon precursor and surface passivation agent and the presence of saccharide is unnecessary.13 Thus, they fabricated carbon nanodots by heating a mixer of PEG-200 and water (with 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio) in a 900 W microwave for 10 min and showed the obtained CND (denoted as CNDA) have low cytotoxicity and tunable, pH independent and stable photoluminescence (PL).13 So, we have decided to study the role of water in the synthesis of carbon nanodots and then optimize the microwave irradiation time and power to obtain a CND sample with high absorption (and consequently high concentration) and fluorescence intensity and low energy consumption.
1 volume ratio) in a 900 W microwave for 10 min and showed the obtained CND (denoted as CNDA) have low cytotoxicity and tunable, pH independent and stable photoluminescence (PL).13 So, we have decided to study the role of water in the synthesis of carbon nanodots and then optimize the microwave irradiation time and power to obtain a CND sample with high absorption (and consequently high concentration) and fluorescence intensity and low energy consumption.
Metronidazole (MNZ), a nitroimidazole derivative, is one of the important drugs used clinically in the treatment of bacterial infections and diseases caused by protozoa.14,15 It has also been utilized as a promoter to stimulate the growth of cattle pigs, poultry and improve feed efficiency.16 Beside of these benefit, MNZ is toxic to the nervous system and has carcinogenic and mutagenic side effects16,17 and use of it in food-producing species has been banned by many countries.15 Therefore, good analytical methods for the determination of drug residues are urgently needed to ensure human food safety and protect the environment. The chemical structure of MNZ, represented in Fig. S1, ESI,† contains a nitro group which is an active reducible center.18 It seems that the active site of MNZ can attract with the surface functional groups of CND and affect the PL property of CND. There are a few reports concerning the detection of MNZ based on quenching property of MNZ.19 Therefore, in the last section of this research work, the feasibility of analytical application of the prepared CND in a system containing metronidazole was investigated based on the fluorescence quenching method.
As mentioned before, CNDA were synthesized via microwave heating of a mixer of PEG-200 and water.13 Therefore, to clarify the factors influencing carbon nanodots formation, the effect of water, one of the variables in this system, was firstly investigated. For this purpose, two samples of CND were synthesized in the same conditions, except for the presence or absence of water. The prepared solutions were diluted with distilled water, then, UV-Visible and fluorescence spectra (exited in λex = 330 nm) were recorded with respect to distilled water as a reference to eliminate the effect of water as the solvent (Fig. 1 and 2, respectively). Accordingly, difference between the recorded spectra is related to quantity of CNDs formed in the absence and presence of water used for synthesis of CND. Although the remaining volume of these samples at the end of pyrolysis was the same, it can be seen from the results that presence of water decreased the absorbance and fluorescence intensity of obtained CND about 30% but λmax and λem remained unchanged. When sample containing a mixture of PEG-200 and water was exposed to microwave mediated heating, at first the water was evaporated and then carbonization of PEG took place. But about the sample containing sole PEG-200, carbonization started from the initial time of irradiation. So in the first sample, a part of heat produced by microwave was devoted to water evaporation instead of carbonization, which is the reason for the low concentration and low PL intensity of CND formed in the presence of water. Therefore, presence of water in this system is unnecessary and should be eliminated in the synthesis of CND to get the sample containing more CND with higher optical properties.
 | ||
| Fig. 1 Absorption spectra of CND formed in the presence and absence of water. The samples are diluted 100 times with water. | ||
 | ||
| Fig. 2 PL emission spectra of: CND prepared in the presence and absence of water (excited at 330 nm). The samples are diluted 100 times with water. | ||
The higher concentration and higher PL intensity of CND were obtained by elimination of water and only microwave heating of pure PEG-200. The shape and intensity of absorption and PL spectra of the one-substrate prepared carbon nanodots may be affected by changing the time and power of microwave irradiation. Therefore, the effect of these two variables on the optical properties of the prepared CND from pure PEG was investigated. Firstly, to study the effects of irradiation power, 20 ml of PEG-200 was exposed to microwave irradiation with different powers including 400, 540 and 700 W for 2 minutes (the minimum time to produce CND), respectively. By increasing the irradiation power, the color of the obtained CND changed from colorless to dark brown (Fig. S2, ESI†), depicting the formation of more CND. The UV-Visible absorption and PL spectra of CND made at different microwave irradiation conditions were recorded and presented in Fig. 3 and 4, respectively. The higher irradiation power leads to increased absorption and emission intensity but detailed information about absorption and PL spectra indicates that no changes occur in λmax and λem position of the CND. Further optimization was followed by surveying the effect of irradiation time. Therefore, the mentioned experiments were repeated but by 9 and 16 min irradiation. In the longer irradiation times, the effect of power increasing was not positive in the whole range so that absorption intensity is maximum when microwave power is 540 W. Therefore, a microwave pyrolysis power of 540 W was selected as optimized irradiation power in the subsequent studies. Comparing the absorption and emission spectra of CND formed at 540 W but different irradiation times indicated that the longer the processing time, the more CND produced. To ensure this increasing trend, two CND samples were synthesized at the same power (540 W) but longer irradiation times of 20 and 30 min and compared with the sample of CND formed by 16 min microwave irradiation. The result was shown in Fig. S3, ESI.† Surprisingly, extending the microwave irradiation time over than 16 min showed a decrease in absorption intensity and consequently a decrease in the concentration of prepared CND. This decrease in concentration may arise from the destruction of CND caused by long time microwave heating with a high temperature. Hence, the sample prepared by a 540 W microwave heating for 16 min was selected as optimal for further studies and denoted as CNDB.
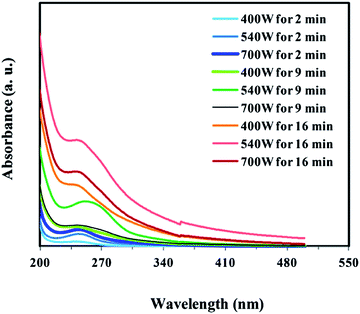 | ||
| Fig. 3 The UV-Visible absorption spectrum of CND made by different microwave treating. The samples are diluted 1000 fold with water. | ||
 | ||
| Fig. 4 PL spectrum of CND obtained by different microwave treating. The samples are diluted 1000 fold with water and excited at 330 nm. | ||
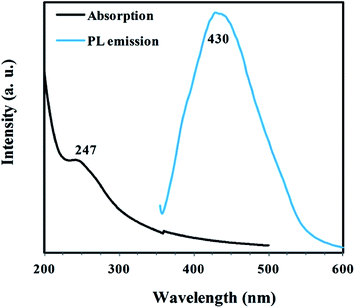
CNDB, the single substrate prepared carbon nanodots, were characterized by different methods. Fig. 5 shows UV-Visible and PL spectra of CNDB diluted with distilled water. UV-Visible spectra showed a peak at 247 nm similar to that observed by Amit and coworker,10,13 which was attributed to the π–π* electronic transitions.
As can be seen in Fig. 5, CNDB revealed an emission peak at 430 nm when excited at 330 nm. This justifies the PL property of CNDB. Note that the PEG has no emission in the visible region. PL spectra of CNDB excited at different wavelengths starting from 320 nm to 440 nm were displayed in Fig. S4, ESI.† It can be seen that PL intensity decreased and emission spectra was shifted to the longer wavelength when the wavelengths of excitation increased. As proposed in previous reports, this red shifted emission pattern (wavelength-dependent phenomena or multicolor PL) can be attributed to the quantum effects (particle of different sizes) and/or presence of emissive traps site on the CND.3,7,11 A small drop of CND solution which was excited under UV light of photoluminescence spectroscopy showed blue photoluminescence (Fig. 6a). TEM image, displayed in Fig. 6b, exhibits the formation of small CNDB particles with the size of less than 5 nm. XRD pattern of CNDB (Fig. S5, ESI†) represents a peak at 2θ = 19° which emphasize on amorphous nature.3 Finally, FTIR spectra were used to recognize the functional groups of CNDB. A comparison between CNDB IR spectra (Fig. S6, ESI†) and PEG-200 IR spectra (data not included) showed that two new peaks at 1601 cm−1 and 1715 cm−1, corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups respectively, appeared as a result of carbonization of PEG-200 by microwave heating. This observation corroborated the UV-Visible results.
O groups respectively, appeared as a result of carbonization of PEG-200 by microwave heating. This observation corroborated the UV-Visible results.
 | ||
| Fig. 6 (a) The photoluminescence image of CNDB under the UV light of photoluminescence spectroscopy. (b) TEM image of the prepared CNDB. Arrows point to some of CNDB shown in the image. | ||
Amit and coworker showed that CND prepared by microwave heating of a mixer of water and PEG-200 have stable PL in the pH range 3–8.13 PL spectra of CNDB were recorded in the acidic, alkaline and natural solutions and also in the presence of NaCl (with body concentration). Fig. 7 shows that the PL intensity of the solutions containing acid, alkali or NaCl remained almost constant with respect to that of natural solution. These observations suggest potential applications of these single substrate prepared carbon nanodots in different mediums.
 | ||
| Fig. 7 PL Emission spectra (excited at 330 nm) of CNDB in the natural, acidic, alkaline solution and in the presence of NaCl. | ||
In order to evaluate the potential application of CNDB, the fluorescence quenching effect of MNZ on the PL intensity of CNDB was studied. From the results shown in Fig. 8a, it was proved that MNZ quenched the PL of CNBD significantly. The aforementioned PL property of CND was attributed to the energy trapping of the organic groups. After MNZ was added to CND, interaction of MNZ with surface functional groups could decrease the PL intensity. As seen from Fig. 8a and b, the PL intensity of CNDB at 430 nm decreased with increasing MNZ concentration. A linear relationship of ln(F0/F) and MNZ concentration was observed within the range of 1–100 ppm with slope of 0.043 ppm−1, in which F0 and F represent the emission intensities of CNDB in the absence and presence of metronidazole (the inset in Fig. 8b). This plot indicates that CNDB solution can be used to quantitatively determine the MNZ. Some of metal ions may interact with surface functional groups of CND and affect the PL intensity of CND.20 Determination of MNZ is important in real samples such as blood plasma21 where the K+, Na+ and Ca2+ are the most ionic constituents.22 These ions may act as contaminants in MNZ detection. Effect of these metal ions on the PL intensity of CNDB in the absence and presence of MNZ was studied (Fig. S7, ESI†). It was found that the presence of these metal ions (in the blood plasma concentration22) has no significant effect on PL intensity of CNDB. This is a good result from the practical point of view and therefore, CNDB can be used as a fast, facile, green and cheap MNZ detector based on fluorescence quenching.
Conclusions
In summary, we produced one-step and one-substrate CND with the high yield and strong optical properties by elimination of water and optimization of power and time of microwave irradiation. Optimum conditions for preparation this single substrate prepared CND are 16 min microwave irradiation of 540 W. It was fond that PL intensity of CND is stable in acidic and alkali mediums and also in the presence of sodium chloride. The prepared CND showed interesting results in the detection of MNZ. Therefore, a fast, facile, low-cost, and eco-friendly method was reported for the synthesis of carbon nanodots with potential application in MNZ detection.Acknowledgements
Financial support from Bu-Ali Sina University is gratefully acknowledged. Acknowledge to Prof. T. Madrakian and Prof. A. K. Chehregani for their technical support.Notes and references
- X. Wang, K. Qu, B. Xu, J. Ren and X. Qu, J. Mater. Chem., 2011, 21, 2445 RSC.
- Z. Chen, J. Chen, Q. Liang, D. Wu, Y. Zeng and B. Jiang, J. Lumin., 2014, 145, 569 CrossRef CAS PubMed.
- H. Zhu, X. Wang, Y. Li, Z. Wang, F. Yang and X. Yang, Chem. Commun., 2009, 5118 RSC.
- D. Xiao, D. Yuan, H. He and J. Lu, Luminescence, 2013, 28, 612 CrossRef CAS PubMed.
- K. Hola, A. B. Bourlinos, O. Kozak, K. Berka, K. M. Siskova, M. Havrdova, J. Tucek, K. Safarova, M. Otyepka and E. P. Giannelis, Carbon, 2014, 70, 279 CrossRef CAS PubMed.
- S.-T. Yang, X. Wang, H. Wang, F. Lu, P. G. Luo, L. Cao, M. J. Meziani, J.-H. Liu, Y. Liu and M. Chen, J. Phys. Chem. C, 2009, 113, 18110 CAS.
- C. Liu, P. Zhang, F. Tian, W. Li, F. Li and W. Liu, J. Mater. Chem., 2011, 21, 13163 RSC.
- J. Jiang, Y. He, S. Li and H. Cui, Chem. Commun., 2012, 48, 9634 RSC.
- H. Peng and J. Travas-Sejdic, Chem. Mater., 2009, 21, 5563 CrossRef CAS.
- X. Zhai, P. Zhang, C. Liu, T. Bai, W. Li, L. Dai and W. Liu, Chem. Commun., 2012, 48, 7955 RSC.
- S. Mitra, S. Chandra, T. Kundu, R. Banerjee, P. Pramanik and A. Goswami, RSC Adv., 2012, 2, 12129 RSC.
- A. Philippidis, D. Stefanakis, D. Anglos and D. Ghanotakis, J. Nanopart. Res., 2013, 15, 1 Search PubMed.
- A. Jaiswal, S. S. Ghosh and A. Chattopadhyay, Chem. Commun., 2012, 48, 407 RSC.
- M. Vosough and H. Mashhadiabbas Esfahani, Talanta, 2013, 113, 68 CrossRef CAS PubMed.
- M. Wagil, J. Maszkowska, A. Białk-Bielińska, M. Caban, P. Stepnowski and J. Kumirska, Chemosphere, 2014 DOI:10.1016/j.chemosphere.2013.12.061.
- D. Chen, J. Deng, J. Liang, J. Xie, C. Hu and K. Huang, Sens. Actuators, B, 2013, 183, 594 CrossRef CAS PubMed.
- W. Tian, L. Gao, Y. Zhao, W. Peng and Z. Chen, Anal. Methods, 2013, 5, 1283 RSC.
- W. Liu, J. Zhang, C. Li, L. Tang, Z. Zhang and M. Yang, Talanta, 2013, 104, 204 CrossRef CAS PubMed.
- A. Muhammad, T. Muhammad, O. Yimit and B. Yakup, J. Fluoresc., 2013, 23, 599 CrossRef CAS PubMed.
- A. Philippidis, D. Stefanakis, D. Anglos and D. Ghanotakis, J. Nanopart. Res., 2013, 15, 1414 CrossRef.
- A. Menelaou, A. A. Somogyi, M. L. Barclay and F. Bochner, J. Chromatogr. B: Biomed. Sci. Appl., 1999, 731, 261 CrossRef CAS.
- H. A. Krebs, Annu. Rev. Biochem., 1950, 19, 409 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, chemical structure of MNZ, photograph of prepared CNDB, the absorption spectrum of CNDB made by different intervals irradiation, PL emission spectra of CNDB exited at different wavelength, XRD pattern and FT-IR spectrum of CNDB. See DOI: 10.1039/c4ra06928e |
| This journal is © The Royal Society of Chemistry 2014 |