Composite cathode material β-LiVOPO4/LaPO4 with enhanced electrochemical properties for lithium ion batteries
Chao Shen,
Jun-chao Zheng*,
Bao Zhang*,
Ya-dong Han,
Jia-feng Zhang,
Lei Ming,
Hui Li and
Xin-bo Yuan
School of Metallurgy and Environment, Central South University, Changsha 410083, P. R. China. E-mail: jczheng@csu.edu.cn; csuzjc@gmail.com; Tel: +86-731-88836357
First published on 14th August 2014
Abstract
A composite cathode material, β-LiVOPO4/LaPO4, is synthesized by a sol–gel method. The synthesized samples are characterized by XRD, SEM, TEM, EDS, XPS, and electrochemical tests. Results indicate that LiVOPO4 has an orthorhombic structure with a Pnma space group and that LaPO4 has a monazite structure with a P21/n space group. EDS and TEM results illustrate that LaPO4 with typical sizes of 10–40 nm is homogeneously distributed on the surface of primary LiVOPO4 particles. The synthesized β-LiVOPO4/LaPO4 exhibits much better electrochemical performance than bare β-LiVOPO4. The β-LiVOPO4/LaPO4 samples delivered an initial discharge capacity of about 127.0 mA h g−1 at 0.1 C and possessed favorable capacities at rates of 0.5 and 1 C. Therefore, surface modification of crystalline LaPO4 is an effective way to improve the electrochemical performance of β-LiVOPO4.
Introduction
Lithium-ion batteries are rechargeable batteries that can be used in portable electronic devices, electric vehicles, and hybrid electric vehicles, among others, because of their high specific capacity, long cycle life, and high operating voltage.1,2 A significant amount of research has focused on new cathode materials for lithium-ion batteries. After Goodenough's laboratory3–5 published their research results, interest in polyanionic compounds as lithium storage electrodes for rechargeable batteries grew. Specifically, lithium transition metal phosphates, such as LiMPO4 (M = Fe, Mn, or Co),6–8 Li3V2(PO4)3 (M = V, Fe, or Ti),9–11 LiVPO4F,12,13 and LiVOPO4,14 have gained extensive attention as cathode materials because of their high energy density, low cost, and excellent thermal stability.LiFePO4 is the simplest, most widely studied, and potentially most useful of these cathode materials because of its high lithium intercalation voltage (3.5 V vs. Li), high theoretical capacity of 170 mA h g−1, and low cost.15 Compared with LiFePO4, lithium vanadyl phosphate (LiVOPO4) exhibits higher energy density at nearly the same theoretical capacity of 166 mA h g−1 and a higher lithium intercalation potential of about 4 V.14 LiVOPO4 exists as α-LiVOPO4 (triclinic, space group ![[P with combining macron]](https://www.rsc.org/images/entities/i_char_0050_0304.gif) 1) or β-LiVOPO4 (orthorhombic, space group Pnma), which exhibit electronic conductivities of about 10−11 and 10−10 S cm−1, respectively.16,17 The orthorhombic phase has been extensively investigated because of its excellent ion-intercalation properties. However, the poor electronic and ionic conductivity has been an obstacle to the development of LiVOPO4.18,19
1) or β-LiVOPO4 (orthorhombic, space group Pnma), which exhibit electronic conductivities of about 10−11 and 10−10 S cm−1, respectively.16,17 The orthorhombic phase has been extensively investigated because of its excellent ion-intercalation properties. However, the poor electronic and ionic conductivity has been an obstacle to the development of LiVOPO4.18,19
Carbon coating, nano-sizing, porous structures, and morphology tailoring have been investigated to improve the performance of LiVOPO4 cathode materials; however, these techniques present certain disadvantages.20–22 Pure-phase LiVOPO4, for example, is synthesized mainly through sintering in air, which results in negative effects on carbon coating and metal doping and leads to poor electrochemical performance. Hence, exploring other novel ideas to realize improved Li battery performance is necessary.
Introducing electrical additives to the surface of cathode materials is an effective way to improve the electrochemical performances of phosphate-based cathode materials.23–25 M. M. Ren synthesized β-LiVOPO4/RuO2 composites presenting good electrochemical Li ion intercalation performance because of enhanced electrical conductivity and Li ion diffusion.20 However, the electrical additives were only coated at a micro-scale level onto the pristine β-LiVOPO4 particles so that improvements in the electrochemical performance of the lithium batteries were not very obvious. According to the ref. 26 introducing LaPO4 (with good ionic conductivity) to the surface of a cathode material (Li[Ni0.5Co0.2Mn0.3]O2) is an effective way to improve the electrochemical performance of Li[Ni0.5Co0.2Mn0.3]O2.
In the present study, β-LiVOPO4/LaPO4 composite was synthesized and characterized, and the effects of LaPO4 surface modification on the structural and electrochemical properties of the composite were investigated.
Experimental
The β-LiVOPO4/LaPO4 composite was synthesized via a sol–gel method. First, a stoichiometric amount of NH4VO3 (AR, ≥ 99.0%), LiNO3 (AR, ≥ 99.0%), (NH4)2HPO4 (AR, ≥ 99.0%), and La(NO3)3(AR, ≥ 99.0%) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05
1.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.03
1.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 molar ratio were dissolved in water with magnetic stirring at 85 °C for 2 h. When the raw materials were completely dissolved, C2H2O4·2H2O (AR, ≥ 99.0%) was dissolved as both the chelating agent and reducer. Next, the mixture was continuously stirred at 85 °C until a dark blue gel formed. This gel was then dried and sintered at 450 °C for 8 h in air atmosphere to form β-LiVOPO4/LaPO4 composite powder. The product was spontaneously cooled to room temperature, and the β-LiVOPO4/LaPO4 composite was obtained. Bare β-LiVOPO4 was synthesized via the same method but without La(NO3)3. The molar ratio of NH4VO3, LiNO3, and (NH4)2HPO4 used was 1
0.03 molar ratio were dissolved in water with magnetic stirring at 85 °C for 2 h. When the raw materials were completely dissolved, C2H2O4·2H2O (AR, ≥ 99.0%) was dissolved as both the chelating agent and reducer. Next, the mixture was continuously stirred at 85 °C until a dark blue gel formed. This gel was then dried and sintered at 450 °C for 8 h in air atmosphere to form β-LiVOPO4/LaPO4 composite powder. The product was spontaneously cooled to room temperature, and the β-LiVOPO4/LaPO4 composite was obtained. Bare β-LiVOPO4 was synthesized via the same method but without La(NO3)3. The molar ratio of NH4VO3, LiNO3, and (NH4)2HPO4 used was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05
1.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
The elemental composition of the powders was determined by XPS on an instrument (Kratos Model XSAM800) equipped with a Mg Kα achromatic X-ray source (1235.6 eV) and a JEOL-energy dispersive spectroscopy (EDS) detector. Structural and crystalline phase analyses of the products were conducted by XRD (Rint-2000, Rigaku) using Cu Kα radiation. The samples were observed by SEM (JEOL JSM-5600LV), and TEM (Tecnai G12). Elemental carbon analysis was performed using C–S analysis equipment (Eltar, Germany).
Electrochemical characterizations were performed using CR2025 coin-type cells. The composite electrodes were prepared by mixing the as-synthesized composite with carbon black and polyvinylidene difluoride at a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 using NMP as the solvent. The cathode was prepared by spreading the mixture on an aluminum foil. Charge–discharge tests of the samples were performed in coin cells with cathodes and lithium anodes. After solvent evaporation, the electrodes were punched to form disks with 14 mm diameter and then dried at 120 °C for 4 h. The test cell consisted of the positive electrode and lithium foil negative electrode separated by a porous polypropylene film, and 1 mol L−1 LiPF6 in EC
10 using NMP as the solvent. The cathode was prepared by spreading the mixture on an aluminum foil. Charge–discharge tests of the samples were performed in coin cells with cathodes and lithium anodes. After solvent evaporation, the electrodes were punched to form disks with 14 mm diameter and then dried at 120 °C for 4 h. The test cell consisted of the positive electrode and lithium foil negative electrode separated by a porous polypropylene film, and 1 mol L−1 LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC
EMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC (1
DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as the electrolyte. The coin cells were assembled in a dry Ar-filled glove box. The cells were charged and discharged over a voltage range of 3.0–4.5 V versus a Li/Li+ electrode at 25 °C. The cycling and charge–discharge performance of the cells was tested using an automatic galvanostatic charge–discharge unit (LAND battery cycle). Cyclic voltammograms were recorded with a scan rate of 0.1 mV s−1 between 3.0 and 4.5 V.
1 v/v) as the electrolyte. The coin cells were assembled in a dry Ar-filled glove box. The cells were charged and discharged over a voltage range of 3.0–4.5 V versus a Li/Li+ electrode at 25 °C. The cycling and charge–discharge performance of the cells was tested using an automatic galvanostatic charge–discharge unit (LAND battery cycle). Cyclic voltammograms were recorded with a scan rate of 0.1 mV s−1 between 3.0 and 4.5 V.
Results and discussion
The XRD patterns of the synthesized powders are shown in Fig. 1. The sharp peaks in the patterns indicate that the powders are well crystallized. The pattern of the β-LiVOPO4 powders is consistent with the JCPDS data (PDF#85-2438) and literature reports.5,25 The main crystalline phase in both samples represents an orthorhombic structure with a Pnma space group. A subtle difference may be observed between bare β-LiVOPO4 and β-LiVOPO4/LaPO4 from 28° to 31° (marked with an oval). The main planes of (1 2 0), (2 1 0), (0 1 2), and (−1 1 2) indicate the diffraction peaks of impurities corresponding to the peaks for LaPO4, which is consistent with the JCPDS data (PDF#83-0651). These results indicate that LaPO4 has a crystalline phase and a monazite structure with a P21/n space group. The weight percentage of LaPO4 in the β-LiVOPO4/LaPO4 composite is 4%. No other diffraction peak is found in the XRD patterns. The amount of carbon in the composites is about 1.2 wt%, as determined by the C–S analysis method. Carbon remaining in the composite could not be detected by XRD, which indicates that the residual carbon is amorphous.SEM images of bare β-LiVOPO4 and β-LiVOPO4/LaPO4 are shown in Fig. 2. The surfaces of bare β-LiVOPO4 particles [Fig. 2(a) and (b)] are slightly smoother than those of β-LiVOPO4/LaPO4 [Fig. 2(c) and (d)]. In addition, the particle sizes of β-LiVOPO4/LaPO4 sample are much smaller than those of bare β-LiVOPO4, which is beneficial to intercalation and de-intercalation of Li ions over short paths. Moreover, β-LiVOPO4/LaPO4 has more pores than β-LiVOPO4; these pores cause an increase in the specific area of the former. The specific surface areas of β-LiVOPO4 and β-LiVOPO4/LaPO4 are 3.012 and 3.898 m2 g−1, respectively.
HRTEM images of β-LiVOPO4/LaPO4 are shown in Fig. 3. As seen in Fig. 3(a) and (b), the β-LiVOPO4 particle is surrounded by LaPO4 nanoparticles, and the particle size of LaPO4 is within 10–40 nm. The presence of LaPO4 as observed in the present study is different from that described in previous literature reports, in which LaPO4 may be observed as a thin amorphous layer on the surface of the particles.25 To verify the characteristics of LaPO4 modification, local amplification of one of the modification sites (marked with a blue circle) was performed, as shown in Fig. 3(c). One type of lattice fringe was found in the LaPO4 modification site [right section in Fig. 3(c)]. The lattice fringe is attributed to LaPO4 with an inter-planar spacing of 3.12 Å, which corresponds to the (1 2 0) lattice plane. These results indicate that crystalline-phase LaPO4 particles are distributed on the surface of β-LiVOPO4 particles, which is consistent with the XRD results. Fig. 3(d) and (e) reveal the Fourier transform (FFT) of the selected areas. The FFT image in Fig. 3(d) shows the diffraction spots of β-LiVOPO4, while the FFT image in Fig. 3(e) corresponds to the diffraction spots of LaPO4.
 | ||
| Fig. 3 (a and b) TEM image of β-LiVOPO4/LaPO4 composite material (c) the local amplification of one of selected LaPO4 site (d and e) fourier transform (FFT) of the selected areas. | ||
To analyze the uniformity of element distribution in LaPO4, EDS analysis was carried out. Fig. 4 shows the EDS patterns of vanadium, phosphorus, and lanthanum in the β-LiVOPO4/LaPO4 sample. Vanadium and phosphorus are uniformly distributed in the sample, and lanthanum is homogeneously dispersed on the surface of primary particles. These results clearly illustrate that crystalline LaPO4 is homogeneously on the surface of β-LiVOPO4 primary particles.
 | ||
| Fig. 4 X-ray spectroscopy (EDS) analysis of vanadium, phosphorus and lanthanum for β-LiVOPO4/LaPO4 composite material. | ||
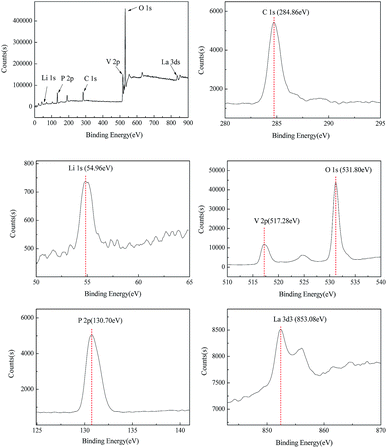
The XPS spectra of β-LiVOPO4/LaPO4 sample are shown in Fig. 5. Peaks appearing at 54.96, 130.70, 531.80, 517.28, and 853.08 eV can be assigned to Li1s, P2p, O1s, V2p, and La3d3, respectively. The test values are fairly consistent with the ref. 27 which reports that Li+, P5+, O2−, and La3+ cations exhibit characteristic peaks of Li1s, P2p, O1s, and La3d3 at 54.9, 130.7, 531.8, and 853.0 eV, respectively. In addition, according to another ref. 28 V4+ (VO2) cations exhibit a peak of V2p at 517.3 eV. These results reveal that the valence state of vanadium in the external surface area of the sample is +4 and that of lanthanum is +3. The results are consistent with the discussion above.
The charge–discharge curves of Li/β-LiVOPO4 and Li/β-LiVOPO4/LaPO4 cells at different rates of 0.1, 0.25, 0.5, and 1 C (160 mA g−1) in the voltage range of 3.0–4.5 V at 25 °C are illustrated in Fig. 6(a). The ΔV of charge–discharge plateaus in the curves of the β-LiVOPO4/LaPO4 cell is much smaller than that of bare β-LiVOPO4. In addition, the initial discharge capacities of β-LiVOPO4 at rates of 0.1, 0.25, 0.5, and 1 C are about 109.1, 94.3, 76.7, and 54.6 mA h g−1, respectively, whereas those of β-LiVOPO4/LaPO4 are about 127.0, 120.0, 103.6, and 83.0 mA h g−1, respectively. Apparently, the rate capacity of β-LiVOPO4/LaPO4 is much better than that of β-LiVOPO4.
Based on Fig. 6(b), the discharge capacities of β-LiVOPO4 at rates of 0.1, 0.25, 0.5, and 1 C are about 99.2, 84.5, 63.3, and 36.0 mA h g−1 after 30 cycles, which indicates that the cell respectively retains 90.9%, 89.6%, 82.5%, and 65.9% of its initial discharge capacity. Compared with β-LiVOPO4, the discharge capacities of β-LiVOPO4/LaPO4 at rates of 0.1, 0.25, 0.5, and 1 C are about 125.0, 116.0, 97.0, and 73.6 mA h g−1 after 30 cycles, and the cell respectively retains 98.4%, 96.7%, 93.6%, and 73.6% of its initial discharge capacity. Thus, the capacity retention of β-LiVOPO4/LaPO4 displays greater advantages compared with that of bare β-LiVOPO4 even at higher rates.
From the discussion above, we can draw the conclusion that the composite cathode material β-LiVOPO4/LaPO4 shows better electrochemical performance than β-LiVOPO4 because of introduction of the ionic conductor LaPO4 to the 3D network between β-LiVOPO4 primary particles.
Fig. 6(c) shows the cyclic voltammetry curves of β-LiVOPO4 and β-LiVOPO4/LaPO4 at a scan rate of 0.1 mV s−1 between 3.0 and 4.5 V. The redox processes of β-LiVOPO4 and β-LiVOPO4/LaPO4 occur at 3.822/4.160 V and 3.839/4.066 V, respectively, which may be assigned to the V4+/V5+ couples and attributed to LiVOPO4. The voltage gap between the redox peaks of β-LiVOPO4/LaPO4 is obviously much smaller than that of bare β-LiVOPO4, which indicates that β-LiVOPO4/LaPO4 presents much better reversibility for Li+ extraction/insertion. These results are in accordance with the charge and discharge curves obtained previously.
Conclusions
β-LiVOPO4/LaPO4 was synthesized via the sol–gel method, and crystalline LaPO4 was successfully on the surface of β-LiVOPO4 primary particles. Introduction of the ionic conductor LaPO4 to the 3D network between β-LiVOPO4 primary particles and the small particle and pore structure of the matrix enhanced the discharge capacity, cyclic stability, and capacity retention of the resultant composite compared with those of bare β-LiVOPO4. Hence, introduction of LaPO4 to β-LiVOPO4 is an effective way to enhance the electrochemical performance of LiVOPO4.Acknowledgements
This study was supported by National Natural Science Foundation of China (Grant no. 51302324 and 51272290) and the Fundamental Research Funds for the Central Universities of Central South University (2013zzts028).Notes and references
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930 CrossRef CAS PubMed.
- H. Li, Z. X. Wang, L. Q. Chen and X. J. Huang, Adv. Mater., 2009, 21, 4953 CrossRef PubMed.
- A. K. Padhi, K. S. Najundaswamy and J. B. Goodenough, J. Electrochem. Soc., 1997, 144, 1188 CrossRef CAS PubMed.
- A. K. Padhi, K. S. Najundaswamy, C. Masquelier, S. Okada and J. B. Goodenough, J. Electrochem. Soc., 1997, 144, 1609 CrossRef CAS PubMed.
- K. S. Nanjundaswamy, A. K. Padhi, J. B. Goodenough, S. Okada, H. Ohtsuka, H. Arai and J. Yamaki, Solid State Ionics, 1996, 92, 1 CrossRef CAS.
- M. Konarova and I. Taniguchi, J. Power Sources, 2010, 195, 3661 CrossRef CAS PubMed.
- D. W. Han, Y. M. Kang, R. Z. Yin, M. S. Song and H. Kwon, Electrochem. Commun., 2009, 11, 137 CrossRef CAS PubMed.
- D. Wang, H. Buqa, M. Crouze, G. Deghenghi, T. Drezenb, I. Exnar, N. Kwon, J. H. Miners, L. Poletto and M. Gratzel, J. Power Sources, 2009, 189, 624 CrossRef CAS PubMed.
- J. C. Zheng, X. H. Li and Z. X. Wang, J. Power Sources, 2010, 195, 2935 CrossRef CAS PubMed.
- A. S. Andersson, B. Kalska, P. Jonsson, L. Haggstrom, P. Nordblad, R. Tellgren and J. O. Thomas, J. Mater. Chem., 2000, 10, 2542 RSC.
- C. M. Burba and R. Frech, Solid State Ionics, 2006, 177, 1489 CrossRef CAS PubMed.
- S. K. Zhong, Z. L. Yin, Z. X. Wang and Q. Y. Chen, J. Cent. South Univ. Technol., 2007, 14, 340 CrossRef CAS PubMed.
- B. Zhang, Y. D. Han, J. C. Zheng, C. Shen, L. Ming and J. F. Zhang, J. Power Sources, 2004, 264, 123 CrossRef PubMed.
- K. L. Harrison and A. Manthiram, Chem. Mater., 2013, 25, 1751 CrossRef CAS.
- J. C. Zheng, X. H. Li, Z. X. Wang, H. J. Guo and S. Y. Zhou, J. Power Sources, 2008, 184, 574 CrossRef CAS PubMed.
- J. Barker, M. Y. Saidi and J. L. Swoyer, J. Electrochem. Soc., 2004, 151, A796 CrossRef CAS PubMed.
- T. A. Kerr, J. Gaubicher and L. F. Nazar, Electrochem. Solid-State Lett., 2000, 3, 460 CrossRef CAS PubMed.
- K. Saravanan, H. S. Lee, M. Kuezma, J. J. Vittal and P. Balaya, J. Mater. Chem., 2011, 21, 10042 RSC.
- D. Morgan, G. Ceder, M. Y. Saidi, J. Swoyer, H. Huang and G. Adamson, Chem. Mater., 2002, 14, 4684 CrossRef CAS.
- M. M. Ren, Z. Zhou, L. W. Su and X. P. Gao, J. Power Sources, 2009, 189, 786 CrossRef CAS PubMed.
- B. M. Azmi, T. Ishihara, H. Nishiguchi and Y. Takita, J. Power Sources, 2005, 146, 525 CrossRef CAS PubMed.
- B. M. Azmi, H. S. Munirah, T. Ishihara and Y. Takita, Ionics, 2005, 11, 402 CrossRef CAS.
- S. H. Lim and J. Cho, Electrochem. Commun., 2008, 10, 1478 CrossRef CAS PubMed.
- P. Mohan and G. P. Kalaignann, Ceram. Int., 1415, 2014, 40 Search PubMed.
- B. Zhang, C. Shen, J. C. Zheng, Y. D. Han, J. F. Zhang, L. Ming, J. L. Wang, S. E. Qin and H. Li, J. Electrochem. Soc., 2014, 161, A748 CrossRef CAS PubMed.
- H. G. Song, K.-S. Park and Y. J. Park, Solid State Ionics, 2012, 225, 532 CrossRef CAS PubMed.
- B. V. Crist, Handbook of Monochromatic XPS Spectra – Vol. 1-The Elements and Native Oxides, XPS International, Inc., 1999 Search PubMed.
- J. C. Zheng, X. H. Li, Z. X. Wang, H. J. Guo, Q. Y. Hu and W. J. Peng, J. Power Sources, 2009, 189, 476 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |