Effect of graphene and carbon nanotubes on the negative active materials of lead acid batteries operating under high-rate partial-state-of-charge operation†
S. Mithin Kumar,
S. Ambalavanan and
Sundar Mayavan*
CSIR-Central Electrochemical Research Institute, Karaikudi, India. E-mail: sundarmayavan@cecri.res.in; Tel: +91 4565241453
First published on 6th August 2014
Abstract
The consequences of including graphene and carbon nanotubes in the negative plates of lead acid batteries have been investigated after exposure to a high rate partial state of charge duty cycle.
The unrelenting issues associated with fossil fuels (like high cost, limited reserves, carbon dioxide emissions) have encouraged the development of a range of hybrid electric vehicles (start–stop hybrid, power-assist hybrid and full hybrid) for transport applications. Lead acid batteries (LAB) in hybrid electric vehicles typically operate under a high rate partial state of charge (HRPSoC). This operating mode is quite challenging as it involves short duration of charge and discharge with high current rates. The regular LAB operating under this mode suffer (or fails prematurely) from two major issues, namely low charge acceptance and progressive sulfation of negative active material (NAM). Creating hybrids/composites structures involving NAM is highly essential to create innovative and intriguing materials as pristine NAM alone cannot satisfy the requirements of LAB operating under HRPSoC.
Carbon addition (to NAM) is considered to be the best strategy to create new functionalities to overcome the mechanism responsible for progressive sulfation. Moseley has explained the possible function of carbon in NAM under HRPSoC conditions.1 Carbon particles improve the electronic conductivity of NAM by forming conductive network between lead sulfate crystals. Presence of carbon may impede the progressive growth of lead sulfate crystals. The capacitive element of carbon can support charge and discharge events at high rates.2 Different types of carbon (with different properties) give rise to different results when added to NAM, some are advantageous and some has no effect.3–5 Hence, active research is currently devoted towards finding an ideal additive for NAM under HRPSoC condition. In this regard, nanostructured graphitic carbon materials offer great promise because of their unusual properties. However, their utilization as NAM additives for LAB operating under HRPSoC condition are rarely studied. Recently, our group found that LAB performance under HRPSoC condition can be improved by replacing conventional carbon black with multiwalled carbon nanotube (MWNT) conductive additive.6 MWNT additives increase the electric conductivity of active mass with sufficient percolation network.
Graphene, a basic building block in all graphitic materials, is a relatively new and exciting material, exhibiting unique electronic, mechanical, and chemical properties.7,8 Recently, graphene has been used as a conductive additive for lithium ion battery cathode materials.9,10 Graphene nanosheets form electron conducting path/network within the cathode thereby improving the capacity and cyclability. In this study we examine the prospects of graphene incorporation (as a conductive additive) into NAM to meet HRPSoC demands. The performance of graphene incorporated in NAM was compared with both single walled carbon nanotube (SWNT) and MWNT incorporated NAM. This study is of great significance as it will allow us to identify the structural changes and elementary process that takes place at the negative plates under HRPSoC cycling and evaluates which types of nano-structured carbon is ideal additive for NAM under our experimental conditions.
In order to evaluate the consequences of including graphene and carbon nanotubes in NAM of LAB during HRPSoC cycling, lead acid test cells (2 V/1.22 A h) were assembled comprising one negative and two positive plates using lead–selenium alloy. The concentration of carbon materials (without any pre-treatment) used as additives in this study is 0.25% by weight. Fig. S1† shows the TEM image of pristine graphene, SWNT and MWNT. Dimensions of negative and positive plates were 40 mm × 49 mm × 1.5 mm and 40 mm × 49 mm × 2.0 mm respectively. The negative lead paste was prepared by conventional method and assembled with PVC separator. The performance of the cells was limited by the negative plate. Firstly, the assembled cells were subjected to 20 h rate cycling (10 cycles) at room temperature. Secondly, the cell performance was evaluated under simulated HRPSoC conditions, using a simplified profile imitating micro-hybrid driving mode.11,12 The cells were subjected to cycling according to the following schedule. Charge at 2 C rate for 60 s, rest for 10 s, discharge at 2 C rate for 60 s, rest for 10 s. The test was stopped when the end voltage reaches 1.83 V or when the upper voltage limit of 2.83 V was reached. The above-described cycling steps comprise of one cycle-set of the test. After this cycle set, the cell was fully re-charged (to 100% SoC) and their C20 capacity was measured then followed to second cycle-set. The charge–discharge tests were carried out using Bitrode life cycle tester. Table 1 and Scheme 1 presents a summary of NAM prepared with three nano-structured carbons and the types of tests and analyses performed within this study.
| Additive | Supplier | Ave. length | Size | Purity | Surface area |
|---|---|---|---|---|---|
| SWNT | Nano shell, USA | 3–8 μm | 1–2 nm (diameter) | >98% | 350–450 m2 g−1 |
| MWNT | SRL, India | 5–15 μm | 40–50 nm (diameter) | >99% | — |
| Graphene | Nano shell, USA | 1–10 μm | 0.5–0.6 nm (thickness) | >99.9% | 250 m2 g−1 |
Fig. 1 shows the FE-SEM images of as-prepared NAM (before cycling) with graphene, SWNT and MWNT additives. Microstructural characterization of graphene–NAM (Fig. 1a–c) reveals globular morphology (spherical) with size of aggregates 1–2 microns. Fig. 1d–i shows that SWNT and MWNT form a network wiring in the NAM. Both SWNT and MWNT were also found to adsorb on the surface of NAM (as seen in Fig. 1f and i). From FE-SEM images it is obvious that graphene and CNTs have different impact on the structural characteristics of the negative active mass. Fig. 2 illustrates the changes in pore radius of NAM with three nano-structured carbon additives (measured by mercury porosimeter). Adding SWNT and MWNT, causes formation of large pores in NAM (2.9 μm and 2.1 μm pore radius, respectively). Addition of graphene reduces the median pore radius to 0.26 μm i.e. negative active mass acquires membrane-sized pore structure as compared to NAM containing SWNT and MWNT. The changes in carbon type in NAM clearly reflect in changes in pore radius distribution.
Fig. 3a shows the comparative cycling performance for cells with graphene, MWNT and SWNT NAM at 20 h rate. Irrespective of type of carbon, the cell capacity decreases during initial cycles and then stabilizes. At the end of 10 cycles, graphene capacity (1.55 A h) is higher as compared to SWNT (1.45 A h) and MWNT (1.4 A h). The cells are then subjected to HRPSoC cycling. Fig. 3b shows the number of completed cycles per cycle-set for three different carbons. The total numbers of completed HRPSoC cycles depend strongly on the nature of carbons. MWNT has completed 7 consecutive cycle-sets with 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 491 cycles as against SWNT and graphene. SWNT failed after 5 consecutive cycle-sets with 26
491 cycles as against SWNT and graphene. SWNT failed after 5 consecutive cycle-sets with 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 644 cycles whereas graphene failed pre-maturely after two consecutive cycle-set with 3874 cycles. Fig. 3c shows the behaviour of the cell capacity (at C20) after each HRPSoC cycle test, the capacity of cells with graphene and SWNT in NAM has decreased dramatically. The cell with MWNT–NAM shows a relatively small decrease in discharge capacity as compared to the initial capacity. These data show that the performance of MWNT–NAM is much better than SWNT and graphene based NAM cell under HRPSoC cycling.
644 cycles whereas graphene failed pre-maturely after two consecutive cycle-set with 3874 cycles. Fig. 3c shows the behaviour of the cell capacity (at C20) after each HRPSoC cycle test, the capacity of cells with graphene and SWNT in NAM has decreased dramatically. The cell with MWNT–NAM shows a relatively small decrease in discharge capacity as compared to the initial capacity. These data show that the performance of MWNT–NAM is much better than SWNT and graphene based NAM cell under HRPSoC cycling.
Failed test cells (containing graphene, MWNT and SWNT) were cut open for analysis. The negative plates were taken out of the cells, washed and dried. Samples were analysed by FE-SEM, XRD, Raman spectroscopy and X-ray photoelectron spectroscopy (XPS). The FE-SEM image of MWNT/SWNT–NAM clearly reveals the distribution of unconverted lead sulfate (PbSO4) crystals. PbSO4 formed on the surface progressively accumulates in the negative plates, which lead to the formation of coarse crystals of irreversible lead sulphate (as shown in Fig. 4e–j). SWNT/MWNT which was firmly adsorbed onto the surface of NAM (before cycling, as seen in Fig. 1f and i) was found to detach itself from the NAM with the formation of coarse PbSO4 crystals. Upon cycling, NAM undergoes large volume change (about 120%), this volume change can result in the detachment of SWNT/MWNT, losing its efficient electronic contact with the NAM. Once SWNT/MWNT is detached, the process of converting PbSO4 to Pb becomes increasingly difficult and may lead to progressive sulfation. Chemical composition and phases present on the electrode surfaces was analysed by XRD (Fig. 5a). XRD data indicates the formation of PbSO4 diffraction peaks along with lead peak in case of MWNT and SWNT.2 This is an indication that MWNT/SWNT–NAM is sulphated more in HRPSoC condition. Interestingly, for graphene–NAM lead oxide (PbO) crystals are formed instead of PbSO4. FE-SEM image (Fig. 4a–c) shows the presence of leaf-like crystals (all over the surface) which is indicative of the presence of PbO crystal and impedes significantly the reaction of lead oxidation. XRD confirms the presence of considerable amounts of PbO along with small amount of PbSO4. The diffraction peaks for PbO are higher than that of metallic lead, which indicates that a thick PbO layer has formed. The formation of PbO (or conversion of Pb to PbO) under HRPSoC duty cycle may be the reason for pre-mature failure of the cell. Fig. 5b–d shows the Raman spectroscopy of MWNT, SWNT and graphene based NAM. All samples display prominent bands at around 1300, 1600 and 2700 cm−1, which corresponds to D, G and 2D band of graphitic materials, respectively.13 The intensity ratio of the D to G bands (ID/IG ratio, which is a measure of the degree of disorder (or defect density)) of NAM–graphene decreases. The presence of H2SO4 (under test condition) may facilitate the conversion of sp3 to sp2 graphitic carbon14 (removal of defects) and hence the decrease. In addition, NAM with both SWNT and MWNT show an additional peak at 978 cm−1. The Raman peak at 978 cm−1 is assigned to the symmetric stretching vibrational mode of the sulfate group in PbSO4.15 But NAM with graphene does not show any peak relating to PbSO4. Fig. 5e compares the Pb 4f peaks of NAM with graphene, SWNT and MWNT. The Pb 4f spectra of graphene–NAM shows two broad singlet peaks corresponding to Pb 4f7/2 and Pb 4f5/2 centered around binding energy values 138.5 eV and 143 eV respectively. No sulphur peak is evident. This indicates that majority of lead appears to be present as lead oxide. In case of SWNT a clear blue shift in peak is observed which indicates the presence of lead as lead sulfate. In addition the sample show S 2p peak at 168.5 eV (Fig. 5f) which is assigned to sulphur species present in PbSO4.16 The Fig. 5g shows the C 1s peak of NAM with three different carbon additives. All samples show a major peak at 285.2 eV with a small shoulder at 289 eV. According to literature the peak at 285.2 eV correspond to carbon attached to hydrogen atom (C–H; BE = 285.2 eV).17,18 The formation of C–H species indicates attachment of hydrogen to the C–C π-bonds resulting in C–H bond formation during HRPSoC cycling. In the present case, C–H formation may be due to intercalation/adsorption of H+ ions (from electrolyte H2SO4). It has been well documented that it is possible to form local C–H bonds by chemical interaction between hydrogen and SWNT/MWNT/graphene.19,20 The small shoulder at 289.6 eV can be assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in carboxylic acid, ester and lactone functionalities.21 The formation of C
O in carboxylic acid, ester and lactone functionalities.21 The formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O may be due to oxidation of carbon under acidic conditions and also by oxygen from positive plate (see ESI†).22
O may be due to oxidation of carbon under acidic conditions and also by oxygen from positive plate (see ESI†).22
 | ||
| Fig. 4 FE-SEM image of cycled NAM (after cell failure) with (a–c) graphene, (d–f) MWNT and (g–i) SWNT. | ||
XPS, XRD and Raman data clearly suggest the formation of PbSO4 and PbO/OH phases in case of SWNT–NAM and graphene/MWNT–NAM, respectively. Pavlov et al.2 had studied the formation of PbO in H2SO4, and has explained the mechanism for the formation of PbO in NAM using porometric data. NAM with narrow pores (less than 1.0 μm) acts as a semi-permeable membrane for ionic transport, since SO42− and HSO4− ions are relatively big in size and less mobile, their movement through small pores may be impeded and pores are filled only with water. Water dissociation and H+ ion migration leaves OH− ions (in pores), and thus increase the pH of the pore solution to alkaline values. A reaction between Pb2+ and OH− ions starts, producing Pb(OH)2 and PbO. Thus, PbO is formed despite active lead is in contact with H2SO4.
We observed a similar trend toward formation of PbO in our present study. Scheme 2 illustrates the process leading to formation of PbO in graphene–NAM. Our porometric data give pore radius values of 2.9 μm, 2.1 μm and 0.26 μm for NAM with SWNT, MWNT and graphene, respectively. NAM–graphene with narrow pores may act as a semi-permeable membrane and blocks/impedes the movement of large size SO42− and HSO4− ions into pores. Thus the solution inside pores becomes alkaline resulting in the formation of PbO. Moreover, wide variety of species can be intercalated between graphene sheets1,18,19 and hence the intercalation of ions (H+)/(HSO4−) and molecules (H2SO4) is also possible (impeding the interaction of NAM with H2SO4).
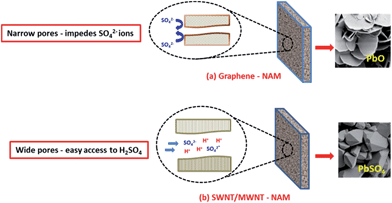 | ||
| Scheme 2 Model scheme for the PbO and PbSO4 formation in NAM pores with graphene and SNT/MWNT, respectively. | ||
Conclusions
This study allowed us to identify the elementary process that take place at the negative plates on HRPSoC cycling. Graphene and CNTs have different impact on the structural and electrochemical properties of NAM. Under HRPSoC cycling, MWNT shows much better activity and performance than SWNT and graphene based NAM. For SWNT–NAM, the dominating elementary process is the formation of PbSO4 on the electrode surface. But for graphene–NAM, the dominating elementary process is the formation of PbO on the electrode surface (which impedes significantly the reaction of lead oxidation and results in pre-mature cell failure as compared to SWNT and MWNT).Acknowledgements
This work was supported by Council of Scientific and Industrial Research (CSIR), India - TAPSUN project NWP 56A.Notes and references
- P. T. Moseley, J. Power Sources, 2009, 191, 134 CrossRef CAS PubMed.
- D. Pavlov and P. Nikolov, J. Power Sources, 2013, 242, 380 CrossRef CAS PubMed.
- K. Nakamura, M. Shiomi, K. Takahashi and M. Tsubota, J. Power Sources, 1996, 59, 153 CrossRef CAS.
- M. Shiomi, T. Funato, K. Nakamura, K. Takahashi and M. Tsubota, J. Power Sources, 1997, 64, 147 CrossRef CAS.
- D. Pavlov, P. Nikolov and T. Rogachev, J. Power Sources, 2011, 196, 5155 CrossRef CAS PubMed.
- M. Saravanan, P. Sennu, M. Ganesan and S. Ambalavanan, J. Electrochem. Soc., 2013, 160, A70 CrossRef CAS PubMed.
- D. Wei and Y. Liu, Adv. Mater., 2010, 22, 3225 CrossRef CAS PubMed.
- C. N. R. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Angew. Chem., Int. Ed., 2010, 48, 7752 CrossRef PubMed.
- G. Kucinskis, G. Bajars and J. Kleperis, J. Power Sources, 2013, 240, 66 CrossRef CAS PubMed.
- C. Su, X. Bu, L. Xu, J. Liu and C. Zhang, Electrochim. Acta, 2012, 64, 190 CrossRef CAS PubMed.
- D. Pavlov, P. Nikolov and T. Rogachev, J. Power Sources, 2010, 195, 4444 CrossRef CAS PubMed.
- D. P. Boden, D. V. Loosemore, M. A. Spence and T. D. Wojcinski, J. Power Sources, 2010, 195, 4470 CrossRef CAS PubMed.
- S. Mayavan, J. B. Sim and S. M. Choi, J. Mater. Chem., 2012, 22, 6953 RSC.
- W. Gao, L. B. Alemany, L. Ci and P. M. Ajayan, Nat. Chem., 2009, 1, 403 CrossRef CAS PubMed.
- N. Dupuy and Y. Batonneau, Anal. Chim. Acta, 2003, 495, 205 CrossRef CAS PubMed.
- S. Mayavan, H.-S. Jang, M.-J. Lee, S. H. Choi and S.-M. Choi, J. Mater. Chem. A, 2013, 1, 3489 CAS.
- M. J. Webb, P. Palmgren, P. Pal, O. Karis and H. Grennberg, Carbon, 2011, 49, 3242 CrossRef CAS PubMed.
- M. L. Ng, R. Balog, L. Hornekær, A. B. Preobrajenski, N. A. Vinogradov, N. Mårtensson and K. Schulte, J. Phys. Chem. C, 2010, 114, 18559 CAS.
- V. Tozzini and V. Pellegrini, Phys. Chem. Chem. Phys., 2013, 15, 80 RSC.
- A. Nikitin, X. Li, Z. Zhang, H. Ogasawara, H. Dai and A. Nilsson, Nano Lett., 2008, 8, 162 CrossRef CAS PubMed.
- Y. Wang, X. Tong, X. Guo, Y. Wang, G. Jin and X. Guo, Nanotechnology, 2013, 24, 475602 CrossRef PubMed.
- K. R. Bullock, J. Power Sources, 2010, 195, 4513 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: TEM image, experimental and instrumentation. See DOI: 10.1039/c4ra06920j |
| This journal is © The Royal Society of Chemistry 2014 |





