Highly permeable poly(4-methyl-1-pentyne)/NH2-MIL 53 (Al) mixed matrix membrane for CO2/CH4 separation
Abstract
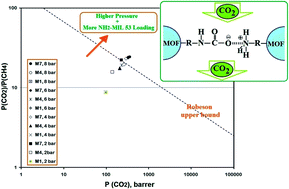
Poly(4-methyl-1-pentyne) (PMP) as a polymer matrix together with synthesized NH2-MIL 53 metal organic framework (MOF) as a filler were used to fabricate a mixed matrix membrane (MMM). Various characterization methods as well as a series of CO2/CH4 gas separation tests (i.e. pure and mixed gas tests) were conducted in order to determine the effect of NH2-MIL 53 on the properties of the prepared MMMs and their gas transport characteristics. The results of TGA and DMA showed that both degradation temperature (Td) and glass transition temperature (Tg) increased by increasing the NH2-MIL 53 loading. SEM images also demonstrated that uniform dispersion of NH2-MIL 53 particles in the PMP matrix was achieved with no noticeable voids in the polymer-filler interfaces. It was also found that incorporation of NH2-MIL 53 in PMP results in an increase of gas permeability (especially for CO2) and higher CO2/CH4 selectivity. In contrast with the increment of CO2 solubility due to the presence of MOF in the polymer matrix, the solubility of CH4 decreases. Although the CO2 solubility was improved with the addition of NH2-MIL 53, its diffusivity remained almost constant with no significant changes. Lastly, it was observed that increasing the MOF loading along with higher feed pressure provide a condition to overcome the Robeson upper bound.

 Please wait while we load your content...
Please wait while we load your content...