Synthesis and bioactivity of nobilamide B†
Abstract
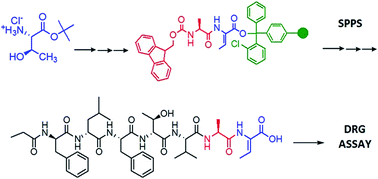
An alternative and facile solution/solid-phase approach is reported for the total synthesis of neuroactive peptide, nobilamide B. Z-Dhb was formed in solution via EDC/CuCl induced elimination. The solid-phase synthesis employed HBTU/Oxyma Pure™ coupling using Barlos resin. Synthetic nobilamide B was also found to be neuroactive in primary cultures of dorsal root ganglion (DRG) neurons.

 Please wait while we load your content...
Please wait while we load your content...