Highly efficient removal of 137Cs in seawater by potassium titanium ferrocyanide functionalized magnetic microspheres with multilayer core–shell structure
Abstract
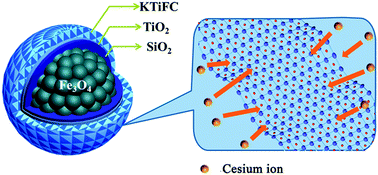
In this study, a novel kind of core–shell-structured magnetic microsphere functionalized with potassium titanium ferrocyanide (KTiFC) was developed for the highly efficient removal of radioactive cesium from seawater. During the synthesis, a compact silica protective interlayer was deliberately constructed to stabilize the nano-sized magnetite cores, while preventing erosion under harsh environmental conditions. Because of high ion exchange capacity of the KTiFC functional layer, the magnetic microspheres exhibited high removal efficiency (≥97.7%) of radiocesium from 137Cs-spiked solutions (3000–35 000 Bq L−1) and contaminated seawater. Batch experiments revealed that adsorption equilibrium was rapidly achieved within 30 min and the maximum adsorption capacity was up to 43.09 mg g−1. Kinetic models and Langmuir/Freundlich adsorption isotherm equations were used to fit the experiment data for describing the adsorption process. Because of the favorable magnetic property, a facile separation and reclamation of the magnetic microspheres from aqueous solution was achieved under an external magnetic field. Moreover, from a practical viewpoint, the magnetic microspheres were proven to have good re-dispersion properties and long-term stability against strong HNO3 solutions (1.0 mol L−1). These magnetic microspheres are believed to hold great promise for the clean-up of radiocesium contaminated water around nuclear facilities and/or after nuclear accidents.

 Please wait while we load your content...
Please wait while we load your content...