Functionalized superparamagnetic Fe3O4 as an efficient quasi-homogeneous catalyst for multi-component reactions†
Abstract
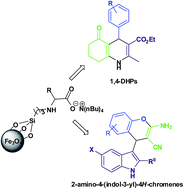
Tetrabutylammonium valinate ionic liquid [NBu4][Val] supported on 3-chloropropyltriethoxysilane grafted superparamagnetic Fe3O4 NPs (VSF) was synthesized and characterized by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), transmission electronic microscopy (TEM), scanning electronic microscopy (SEM), thermal gravimetric analysis (TGA), and vibrating sample magnetometer (VSM). The VSF catalyst was used as an efficient “quasi-homogeneous” catalyst for the multi-component synthesis of 1,4-dihydropyridines and 2-amino-4-(indol-3-yl)-4H-chromenes at room temperature. The VSF catalyst was recovered using an external magnet and recycled six times without a significant loss in the catalytic activity. Moreover, VSF as a “quasi-homogeneous” catalyst can bridge the gap between homogeneous and heterogeneous catalyses.

 Please wait while we load your content...
Please wait while we load your content...