Decoration of homopolymer vesicles by antibacterial ultrafine silver nanoparticles
Abstract
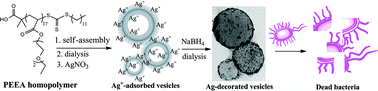
Presented in this article is the proof of principle that silver nanoparticles can be decorated on homopolymer vesicles. The homopolymer vesicles are prepared by self-assembly of poly(2-(2-ethoxyethoxy)ethyl acrylate) (PEEA) in water/tetrahydrofuran (THF) solvent mixture. The –COOH end groups on the surface of the homopolymer vesicle facilitate the growth of ultrafine silver nanoparticles because of the electrostatic interactions between the negatively charged carboxyl group and positively charged Ag+ ions, which were in situ reduced by sodium borohydride (NaBH4). Those silver-decorated PEEA homopolymer vesicles exhibit good antibacterial activity against both Gram-positive and Gram-negative bacteria. This strategy may be extended to prepare more economic antibacterial agents and catalysts.

 Please wait while we load your content...
Please wait while we load your content...