Effect of post-treatment on ordered mesoporous silica antireflective coating
Jinghua Sunab,
Ce Zhangab,
Cong Zhangab,
Ruimin Dinga and
Yao Xu*a
aKey Laboratory of Carbon Materials, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China. E-mail: xuyao@sxicc.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 3rd October 2014
Abstract
Ordered mesoporous silica coating used as an optical antireflective (AR) coating was successfully prepared using tetraethyl orthosilicate as a precursor in the direction of surfactant F127. After various post-treatments, the effect of optical, surface and structural properties on ordered mesoporous silica AR coating was investigated. Ammonia vapor treated ordered mesoporous AR coating showed high transmittance of 99.99% on a quartz substrate. After hexamethyldisilazane (HMDS) treatment, the contact angle with water increased from 7° to 88°. The in situ grazing incident small angle X-ray scattering (GISAXS) was used to investigated the structure evolution of the ordered mesoporous silica coating during the calcination process. The results indicated that the mesopores in the coating constructed a Fmmm orthorhombic symmetry structure with (010) planes parallel to the substrate. The silica network contracted along the direction vertical to the substrate during calcination to remove the template, while in the direction parallel to the substrate, the shrinkage was hindered by the adhesion of the coating. In addition, the Fmmm orthorhombic symmetry structure was maintained after various post-treatments.
Introduction
In order to reduce light reflection, antireflective (AR) coatings have been widely used in optical devices such as solar collector covers and solar cells.1–3 Generally, excellent optical properties, high abrasion-resistance and good environment stability are the most important factors for optical AR coating. Excellent optical properties of AR coating can be obtained using the following principles: the thickness of coating should be λ/4, where λ is the wavelength of the incidence light, and nc = (nans)0.5, where nc, na, and ns are the refractive indices of the coating, air, and substrate, respectively.4 The refractive indices of common optical substrates are about 1.46–1.52, which implies that the refractive index of coating must be about 1.22 to obtain zero reflectance. However, this is a difficult task because the lowest refractive index among one-phase materials is above 1.35.5 The most common way to obtain low refractive index is to use nanoporosity to reduce the refractive indices of materials. Porous silica AR coatings prepared by Stöber method6 are the most commonly used AR coatings because of the excellent transmittance. The coating has low refractive index owing to the voids between the particles, making the transmittance of near 100%. But the coating can be easily irreversibly damaged when physically toughed or wiped.7,8 However, the AR coatings used for some optical equipment such as solar collector covers need strong abrasion-resistance to outdoor use, which means good mechanical stability. For solving this problem, some techniques have been developed. Ammonia vapor was used as a condensation promoter to enhance the mechanical performance of coating,9,10 but the effect is not idealistic. Because the binding force between silica particles and substrate is quite weak as well as between particles in coating. Some works tried to prepare the AR coating via a base/acid two-step catalyzed method which can improve the mechanical property, however, the maximum transmittance is significantly lowered.8,11 In addition, the environment stability of optical AR coating is also a very important issue to take into account. The absorption of water is the most important factor in the outdoor atmosphere condition, which will increase the refractive index and decrease the optical transmittance,8,12 thus the AR coating requires good hydrophobicity.From the viewpoint of practical application, ordered mesoporous silica coating seems to be one of the best candidates as AR coating because it has high porosity, adjustable pore size and excellent mechanical property.13–16 Moreover, the introduction of appropriate organic groups can greatly improve the environment stability of coating.17 High porosity and adjustable pore size afford the coating a low and matched refractive index, further making sure high transmittance of the AR coating. The ordered mesoporous silica coating prepared in acidic catalysis condition has strongly cross-linked pore framework and good adhesion on substrate.18,19 The silica framework modified by organic functional groups can reduce the absorption of water.20,21 The presence of –OH groups causes the coating to adsorb large amounts of water and results in a decrease of coating performance. Therefore, appropriate post-treatment is required to eliminate the remaining silanols and stabilize the mesoporous coating towards moisture absorption. Previously, ammonia vapor treatment has been adopted to stiffen the silica network;22 hexamethyldisilazane (HMDS) has been shown to be effective for the removal of the Si–OH groups in ordered mesoporous silica coating and thus lowered the dielectric constant.23 In ordered mesoporous silica coating, the formation of ordered mesopores during the removal of the template is a dynamic process, the ability to follow the real-time structural evolution of ordered mesoporous silica coating during calcination process becomes desirable. In situ 2D Grazing Incident Small-angle X-ray Scattering (GISAXS) is a powerful tool to investigate the structure of self-assembled nanostructure coatings. But until now, the corresponding work is seldom reported.
Experimental
Preparation and post-treatments of AR coating
The sols used for deposition were synthesized using the following preparation. A stock solution of prehydrolyzed silica precursor was prepared by adding tetraethyl orthosilicate (TEOS, ACROS, 98%), ethanol (Beijing Chemical Works), H2O and HCl (Beijing Chemical Works) with molar ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.006. The sol was left to react under stirring at 60 °C for 1.5 h. A second solution was prepared by dissolving 3.15 g F127 (SIMGA, OH(CH2–CH2O)106(CHCH3CH2O)70(CH2–CH2O)106H) in 67.6 ml of ethanol and 4.5 ml of acid aqueous solution. The final sol was stirred for 24 h and aged for a week at room temperature. The coatings were produced by dip coating at 150 mm min−1 withdrawal rate on quartz substrate which has a diameter of 35 mm and a thickness of 4 mm. Substrates were cleaned in 1 M HNO3 for 12 h at room temperature and successively rinsed with water, ethanol and acetone. After deposition, the as-prepared coating was dried in air for 24 h and then calcined at 350 °C for 3 h to remove template with 5 °C min−1 rate of rise of temperature. Thus-obtained mesoporous silica coating was marked as C. To stiffen of the silica network, the as-prepared coating was left in a saturated ammonia atmosphere for 30 min and then dried for 24 h in air. After calcined at 350 °C for 3 h to remove template, the obtained coating was labeled as CN. To improve the hydrophobicity of mesoporous silica coating, the as-prepared coating was firstly left in a saturated ammonia atmosphere for 30 min and then placed in a closed 500 ml vessel together with a glass container filled with 5 ml HMDS ([(CH3)3SiNHSi(CH3)3], ACROS, 98%) for 24 h at room temperature. After dried for 24 h in air and calcined at 350 °C for 3 h to remove template, the coating was marked as CNH. To further increase the hydrophobicity of coating, the coating CNH was again treated in HMDS vapor for 20 min, and the corresponding coating was CNHH.
0.006. The sol was left to react under stirring at 60 °C for 1.5 h. A second solution was prepared by dissolving 3.15 g F127 (SIMGA, OH(CH2–CH2O)106(CHCH3CH2O)70(CH2–CH2O)106H) in 67.6 ml of ethanol and 4.5 ml of acid aqueous solution. The final sol was stirred for 24 h and aged for a week at room temperature. The coatings were produced by dip coating at 150 mm min−1 withdrawal rate on quartz substrate which has a diameter of 35 mm and a thickness of 4 mm. Substrates were cleaned in 1 M HNO3 for 12 h at room temperature and successively rinsed with water, ethanol and acetone. After deposition, the as-prepared coating was dried in air for 24 h and then calcined at 350 °C for 3 h to remove template with 5 °C min−1 rate of rise of temperature. Thus-obtained mesoporous silica coating was marked as C. To stiffen of the silica network, the as-prepared coating was left in a saturated ammonia atmosphere for 30 min and then dried for 24 h in air. After calcined at 350 °C for 3 h to remove template, the obtained coating was labeled as CN. To improve the hydrophobicity of mesoporous silica coating, the as-prepared coating was firstly left in a saturated ammonia atmosphere for 30 min and then placed in a closed 500 ml vessel together with a glass container filled with 5 ml HMDS ([(CH3)3SiNHSi(CH3)3], ACROS, 98%) for 24 h at room temperature. After dried for 24 h in air and calcined at 350 °C for 3 h to remove template, the coating was marked as CNH. To further increase the hydrophobicity of coating, the coating CNH was again treated in HMDS vapor for 20 min, and the corresponding coating was CNHH.
Characterizations of AR coating
The coating was measured by a Fourier transform-infrared (FTIR) spectrometer (Thermo Scientific, iS50) in the range 500–2500 cm−1. The transmittance of the AR coating was tested with UV-Vis spectrometer (U-4100, Hitachi). The refractive index and physical thickness were measured by a spectroscopic ellipsometer (SE 850, Sentech) at 70° incidence, with a relative measurement error of about ±1%. The surface morphology of the coating was tested using an atomic-force microscopy (AFM, Nanoscope IV, Digital Instruments) and the smallest area of measurement is 5 μm × 5 μm for a large aperture coating. The surface wetting property was measured using a water contact angle goniometer (SL200B). The as-synthesized coating was scratched from the substrate and analyzed by a thermogravimetry (TG, STA-409PC, NETZSCH). The temperature range was from 40 °C to 350 °C and maintained 3 h at 350 °C with a heating rate of 5 °C min−1 in air atmosphere.To investigate the structural evolution of various post-treated ordered mesoporous silica coatings during calcination to remove the surfactant, in situ GISAXS experiment was done at Synchrotron Radiation Facility in China, where the X-ray wavelength was 0.154 nm and the camera length was 1680 mm. To obtain good heat transmission, the coating was dip-coated on silicon substrate. A purpose-built GISAXS furnace consisting of a circular hotplate of 30 mm in diameter was used. The coating was heated from 50 °C, in steps of 100 degrees, up to 350 °C and maintained 1 h and 3 h at 350 °C. After reaching every required calcination temperature, the coating was measured using the time interval 1 s exposure and 3 s detector read-out time. The thermocouple used for temperature control was placed on the silica coating as close as possible to the tested area without interfering with the scattering. A GISAXS pattern was collected at different temperature and a two-dimensional detector with 2048 × 2048 pixels was used to collect both in- and out-of-plane scattering data. Due to the presence of an intense specular beam at grazing angles of incidence, lead strips were used to attenuate the scattering along the specular plane. The incident angle of primary beam to coating surface was chosen at 0.25°. The above procedure was then repeated for the different post-treatment coatings.
Results and discussion
The thermal evolution of AR coating structure
In general, ordered mesoporous silica coating can be synthesized via the hydrolysis–condensation of organic precursors and the self assembled procedure of surfactant molecules. Preferential evaporation of the solvent during dip- or spin-coating process drives cooperative self-assembly from a homogeneous solution of silica and a surfactant dissolved in alcohol below the critical micelle concentration.24 After thermal treatment, the ordered mesopores with various pore structures were formed in coating. Ordered mesopores in coating tend to orient with a specific (hkl) plane parallel to the substrate,25 and it is possible to identify a unit cell of specific dimensions which generates a three-dimensional order. In general, the ordered array of pores in the mesostructured coatings is well described in terms of a “crystalline-like” structure because of the periodic voids in the amorphous network, while the dimensions of the unit cells will be quite larger in comparison with ordinary crystalline materials.26 The ordered mesoporous silica coating prepared by above procedure adheres closely on the quartz substrate and can not be detached from the substrate in nondestructive condition, thus the disturbance from substrate is serious. Moreover, there are very few resolvable peaks in the XRD data from mesoporous coating due to the one-dimensional (1D) scan of reciprocal space in traditional 1D diffractometers. Therefore, it is difficult to identify the structure and lattice constants of mesoporous silica coating from traditional XRD. GISAXS is an ideal tool for the investigation nanostructures assembly and evolution, because it can reveal the most features of reciprocal space. At the grazing incidence angle, the effective coating volume for X-ray scattering becomes large and hence avoids the disturbance from substrates, which will result in strong scattering patterns from coating with only a few hundred nanometers. In this work, considering the removal of template is a dynamic process, in situ GISAXS was used to investigate in detail structural evolution of ordered mesoporous silica coating.The in situ GISAXS images of untreated ordered mesoporous silica coating obtained in a temperature series as the removal of template are shown in Fig. 1. Bragg diffraction spots can be clearly observed from Fig. 1, confirming that the mesoporous silica coating is highly organized. Interpretation of the GISAXS patterns was aided by NANOCELL,27,28 a Mathematica-based program that simulated quantitatively the positions of Bragg peaks at any incidence angle above the critical angle. This simulation program used the distorted wave Born approximation to account for the effects of refraction and reflection at coating-substrate and coating-air interfaces. The hollow circles and squares in the patterns represented the simulated transmissive and reflective spots respectively. The observed and simulated spots were in a good agreement and all the spots were fitted to a face-centered orthorhombic Fmmm structure with (010) planes parallel to the substrate. For the untreated mesoporous silica coating C at 50 °C (Fig. 1a), five diffraction spots were clearly observed and indexed to (111), (131), (151), (242) and (313) reflections of the Fmmm space group. As the removal of template, spots became more obvious, indicating the long periodicity of mesostructure was improved and better than its parent. The pore structure of mesoporous silica coating calcined at temperature 150 °C produced additional diffraction spots (060), (222) and (333) (Fig. 1b), indicating more regularly rearranged pores appeared due to the decrease of the stress on silica wall. With the calcined temperature increased from 250 °C to 350 °C maintaining for 1 h (Fig. 1c–e), (333) spot disappeared, suggesting partly collapse existed in coating. When the mesoporous silica coating was calcined at 350 °C maintaining for 3 h (Fig. 1f), the number of diffraction spots had no change, suggesting a Fmmm structure was maintained. In addition, the patterns were elongated in the vertical direction as the temperature of the additional heat treatment increased, corresponding to the contraction in the vertical direction of the coating. Generally speaking, mesoporous silica coatings with 3D mesoporous structure can be synthesized using PEO-PPO-PEO block polymers as template with EO/PO group ratio higher than 1.5.14 Similar ordered mesoporous silica coatings prepared using triblock copolymer F127 as a template have been reported in some literatures.29–31 Referred to the phase diagrams of Pluronic F127,32 a primitive cubic structure of Im3m symmetry should arise from the arrangement of spherical micelles. Therefore, the Fmmm mesostructure presented here should result from the uniaxial shrinkage along the substrate normal.
The structure parameters of untreated coating C at different calcined temperature are shown in Table 1. The symbol a, b and c were used to mark the lattice constants obtained by GISAXS and NANOCELL fitting. The lattice constants of the untreated coating C at 50 °C were determined to be a = 21.0 nm, b = 22.1 nm and c = 25.0 nm. From Table 1, with increasing the calcined temperature, the constant b decreased from 22.1 nm of 50 °C to 15.8 nm of 350 °C maintaining for 1 h. After calcination at 350 °C maintaining for 3 h, the constant b further decreased to 15.5 nm, due to the contraction of silica network in the direction vertical to substrate during calcination to remove template. However, the constants a and c showed no change, because the shrinkage of coating structure in the direction parallel to substrate had been hindered by the adhesion of coating.
| Temperature | a [nm] | b [nm] | c [nm] | W [%] | ||
|---|---|---|---|---|---|---|
| C | CN | CNH | ||||
| a a, b and c are the lattice constants fitted from 2D GISAXS. W is the retaining weight of mesoporous silica coating from TG result. “/” means that those parameters did not be measured. 350 °C – 1 h means the calcination temperature maintaining for 1 h at 350 °C. 350 oC – 3 h means the calcination temperature maintaining for 3 h at 350 °C. | ||||||
| 50 oC | 21.0 | 22.1 | 22.7 | 23.5 | 25.0 | 99.43 |
| 150 oC | 21.0 | 21.0 | / | / | 25.0 | 97.39 |
| 250 oC | 21.0 | 18.9 | / | / | 25.0 | 80.36 |
| 350 oC | 21.0 | 16.7 | 18.1 | 21.2 | 25.0 | 52.97 |
| 350 oC – 1 h | 21.0 | 15.8 | / | / | 25.0 | 49.11 |
| 350 oC – 3 h | 21.0 | 15.5 | 17.2 | 20.0 | 25.0 | 48.01 |
The structural evolution of ordered mesoporous silica coating related to the decomposition of surfactant F127 during heat treatment was further studied by TG analysis. The organization of silica–surfactant mesophase involving silica frameworks and surfactant regions is formed by sol–gel and dip-coating processes. During the calcination treatment, the surfactant F127 decomposes and the Si–OH group condenses, eventually the contraction of the mesoporous structure occurs.33 The TG curve of untreated mesoporous silica coating containing template F127 is shown in Fig. 2 and corresponding retaining weight at different temperature is shown in Table 1. About 2.04% weight loss from 50 to 150 °C was due to the loss of physically adsorbed water and ethanol, corresponding slight contraction of lattice constant b from 22.1 nm to 21.0 nm. With increasing the calcined temperature, a large weight loss appeared between 97.39% at 150 °C and 52.97% at 350 °C, which was attributed to the decomposition and combustion of surfactant F127 and the condensation of the Si–OH group. After maintaining the temperature at 350 °C from 1 to 3 h, the weight loss was only 1.1%, and the decrease of the lattice constant b was 0.3 nm, indicating the structure had little change. Therefore, the surfactant F127 has been completely removed at 350 °C for 3 h.
Effect of ammonia treatment on AR coating structure
The ordered mesoporous silica coating was formed after heat treatment to removal of template. Many experiments showed mesoporous structure tended to collapse during heat-treatment especially in the present of vapor. To stiffen the inorganic network of silica coating, ammonia vapor was used as a condensation promoter to pre-treat the fresh silica coating with template.22,34 The effect of ammonia treatment can be interpreted by FTIR analysis shown in Fig. 3. There were three absorption peaks could be seen in untreated mesoporous silica coating C (Fig. 3a). The absorption peaks at 1060 and 808 cm−1 were assigned to Si–O–Si antisymmetric and symmetric stretching vibration, which indicated the formation of the silica network. The absorption peak at 947 cm−1 was assigned to stretching vibration of Si–OH. After ammonia treatment, the absorption peak of Si–OH at 947 cm−1 became weaker (Fig. 3b), suggesting that ammonia vapor promoted the condensation of Si–OH groups.The effect of ammonia treatment was further confirmed by GISAXS analysis. Fig. 4 shows the in situ GISAXS images of ammonia-treated coating CN at 50 °C, 350 °C and 350 °C maintaining for 3 h. The results of other calcined temperature at 150 °C, 250 °C and 350 °C maintaining for 1 h were not provide here, because they had similar trend as the untreated coating during calcination process. The same diffraction spots as that of untreated coating C could be seen in three images, suggesting ammonia treatment process was helpful to maintaining the ordered structure. The lattice constants of ammonia-treated coating CN are listed in Table 1. It can be seen that ammonia-treated ordered mesoporous silica coating had the larger lattice constant b than untreated coating at every different calcined temperature. After calcined at 350 °C for 3 h, the constant b is 17.2 nm, still greater than 15.5 nm of untreated coating. The reason is that ammonia vapor treatment process increased the density of silica mesoporous network and decreased the contraction during calcination. However, in the direction parallel to substrate, the lattice constants a and c had no change because of the adhesion of coating.
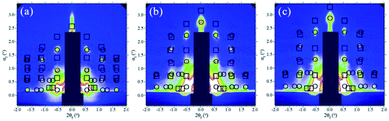 | ||
| Fig. 4 The in situ GISAXS patterns of ammonia-treated coating CN (a) 50 °C, (b) 350 °C, (c) 350 °C maintaining for 3 h. | ||
Effect of HMDS treatment on AR coating structure
After calcination to remove the surfactant, the surface of AR coating appears abundant hydroxyl groups. These hydroxyl groups can easily absorb water in the outdoor atmosphere condition, which will increase the refractive index and decrease the optical transmittance. To increase the hydrophobicity of the AR coating, HMDS was used to treat ordered mesoporous silica coating. The FTIR results of HMDS-treatment are shown in Fig. 3c and d. Compared with ammonia-treated coating CN, a small absorption peak appeared at 1400 cm−1 in the HMDS treated ordered mesoporous silica coating CNH (Fig. 3c), which was assigned to the symmetric bending vibration of the –CH3 group,23 indicating that –Si(CH3)3 groups had substituted for Si–OH groups in coating. In Fig. 3d, the absorption of –CH3 groups became more intense and Si–OH groups were nearly disappeared after twice HMDS treatment due to more Si–OH groups had been replaced with –Si(CH3)3.Fig. 5a–c show the in situ GISAXS images of HMDS-treated coating CNH at 50 °C, 350 °C and 350 °C maintaining for 3 h. The coating CNH had a ring pattern in small angle after heat treatment at 50 °C (Fig. 5a), indicating that the pore structure existed but had a low degree of pore ordering, corresponding to a random pore orientation.35 This indicates that HMDS treatment disordered the pore structure by instituting –Si(CH3)3 groups for –OH groups. The substituted –Si(CH3)3 group had a large volume than the removed –OH group, and the high concentration of –Si(CH3)3 groups induced physical contact between the groups, resulting in stress on the mesoporous silica wall. This stress on the silica wall made the mesoporous structure disordered.23 This ring pattern became more diffuse and was elongated when the heat treatment temperature increased to 350 °C maintaining for 3 h, as shown in Fig. 5c. However, the similar diffraction spots still can be clearly observed and indexed as Fmmm space group, suggesting the fundamental network structure was maintained, even though the surface was modified by ammonia and HMDS treatment. Compared with untreated and ammonia-treated mesoporous silica coating, the ammonia and HMDS treated mesoporous silica coating had the largest lattice constant b at every different calcined temperature (shown in Table 1). The reason is that the chemical modification was followed after the coating synthesized. Plenty of –OH groups were replaced with –Si(CH3)3, therefore there were no much –OH groups, and the coating did not condense too much. Fig. 5d shows the GISAXS pattern of twice HMDS-treated mesoporous silica coating CNHH. The structure and lattice constants had little changed compared with the calcined coating CNH at 350 °C for 3 h. Because the fundamental mesoporous skeleton of ammonia and HMDS treated coating CNH had been solidified after 350 °C calcination for 3 h to remove the surfactant, and thus second HMDS surface modification cannot affect the real structure.
 | ||
| Fig. 5 The in situ GISAXS patterns of HMDS treated coating CNH (a) 50 °C, (b) 350 °C, (c) 350 °C maintaining for 3 h and (d) twice HMDS-treated coating CNHH. | ||
The optical property of AR coating
Fig. 6 shows the transmittance spectra of ordered mesoporous silica AR coatings with various post-treatments on quartz substrate and the corresponding peak transmittance are collected in Table 2. The quartz substrate had a characteristic absorption at 1385 nm. With coating, the transmittance enhanced at least 6% compared to bare quartz substrate. The transmittance of peak value increased from 99.46% of untreated coating C to 99.99% of CN after ammonia-treatment. For the ammonia and HMDS treated coating CNH, the transmittance decreased to 99.85%. After twice HMDS treatment, the transmittance of coating decreased to 99.73%, still maintaining good AR performance.| Coating | Treatment | T [%] | d [nm] | nf | Vp [%] | Rq [nm] |
|---|---|---|---|---|---|---|
| a T is the transmittance at peak value of AR coating. d is the thickness of coating measured by ellipsometer. nf is the refractive index of coating. Vp is the porosity of coating. Rq is the root-mean-square roughness of coating. “/” means the coating has not treatment. | ||||||
| C | / | 99.46 | 198.4 | 1.274 | 37.21 | 3.1 |
| CN | NH3 | 99.99 | 236.3 | 1.229 | 46.91 | 3.9 |
| CNH | NH3 + HMDS | 99.85 | 289.0 | 1.243 | 43.86 | 4.0 |
| CNHH | NH3 + HMDS + HMDS | 99.73 | 295.2 | 1.255 | 41.27 | 8.2 |
Refractive index and thickness of ordered mesoporous silica AR coating were measured with ellipsometer and corresponding results were also shown in Table 2. The refractive index of ammonia-treated coating CN decreased from 1.274 of untreated coating C to 1.229, while the thickness increased from 198.4 nm of C to 236.3 nm. The reason is that ammonia treatment strengthened the mesoporous silica skeleton and reduced the collapse of pore during calcination. Compared with ammonia-treated coating CN, the refractive index of ammonia and HMDS treated coating CNH increased to 1.243, and the thickness increased from 236.3 nm of CN to 289.0 nm, indicating some –Si–OH had been substituted by –Si(CH3)3, and no much condensation of –Si–OH groups during calcination. After twice HMDS treatment, the refractive index had a slight increase and the thickness of coating increased about 6 nm, suggesting more –Si–OH groups were replaced by –Si(CH3)3 groups.
The porosity can be calculated from the refractive index of the coating based on the Lorentz–Lorenz relationship:36
| (nf2 − 1)/(nf2 + 2) = (1 − Vp)(ns2 − 1)/(ns2 + 2) |
The surface morphology of AR coating
The typical morphology and surface roughness of various post-treated ordered mesoporous silica coatings were tested by AFM. The 3D AFM images and root-mean-square roughness, Rq, of all the coatings are shown in Fig. 7 and Table 2. The tested zone of all coatings was 5 μm × 5 μm. Specifically, there were some small particle-like bumps on the surface of the untreated coating C (Fig. 7a) and the Rq is 3.1 nm. These particle-like bumps comes from a higher degree of condensation in the amorphous silica pore walls during thermal treatment, thus they have not particular shape and size. For ammonia-treated mesoporous coatings CN, particle-like bumps increased and became larger (Fig. 7b), and the Rq increased to 3.9 nm, resulted from more condensation Si–OH groups in the coating. The ammonia and HMDS treated coating had similar surface morphology to the coating CN, some larger bumps could be seen in Fig. 7c, and the Rq had a small increase. The reason is that some –Si–OH groups on the surface of coating CNH had been substituted by –Si(CH3)3. After calcination to remove the template, more –OH groups exposed, thus more –CH3 grafted on the coating during twice HMDS treatment. As shown in Fig. 7d, greater particle-like bumps can be seen on the coating CNHH, and the Rq of coating drastically increased. From above surface roughness, all the coatings are all smooth enough to avoid surface scattering which is harmful for antireflection.The hydrophobicity of AR coating
The wettability of liquid to solid surface is governed by the chemical properties of solid surface and its surface morphology. The water contact angles of AR coatings are shown in Fig. 8. The untreated mesoporous coating C demonstrated a water contact angle of 7° (Fig. 8a), as is commonly defined as superhydrophilc. This extreme wetting condition has been attributed to the presence of abundant hydroxyl groups on the surface of silica coating. These hydroxyl groups readily accepted hydrogen bonding with water and contributed to the rapid spreading.37 The contact angle of ammonia-treated mesoporous silica coating CN increased to 10° due to the partly condensation of Si–OH groups (Fig. 8b). For ammonia and HMDS treated coating CNH, the contact angle increased to 38° (Fig. 8c), suggesting some –CH3 groups had been grafted on the surface of the coating CNH although it was calcined at 350 °C for 3 h. FTIR spectrum analysis was also confirmed the existence of –CH3. The surface morphology of coating has a large effect on water repellence. Tadanaga et al.38 reported that water repellence increased with the increase of surface roughness. According to the surface roughness by AFM examination, the ammonia and HMDS treated coating CNH exhibited a little increase of surface roughness. Thus here the surface morphology of the coating made little contribution to the increasing water contact angle. Owing to the reaction of HMDS with hydroxyl groups on the silica wall, the surface was partly covered by methyl groups that improved water repellence.10 The twice HMDS treatment further improved the hydrophobicity of coating (Fig. 8d), and the contact angle had a large increase to 88°.Fig. 9 shows the transmittance spectra of ordered mesoporous silica AR coating C and CNHH at different humidity. The transmittance of AR coating C decreased significantly after maintaining for 6 months in 50% humidity (Fig. 9a), and the peak transmittance decreased from 99.46% to 98.01%. When the coating was put into saturated steam and maintained for 10 min, the peak transmittance decreased to 97.50%. However, the transmittance of AR coating CNHH had little change after maintaining for 6 months in 50% humidity (Fig. 9b), and the peak transmittance decreased from 99.73% to 99.57%. When the coating was put into saturated steam and maintained for 10 min, the peak transmittance decreased to 99.42%. The good stability for humidity of AR coating confirmed the successful hydrophobic modification by HMDS.
 | ||
| Fig. 9 Transmittance spectra of ordered mesoporous silica AR coating at different humidity (a) untreated coating C and (b) twice HMDS treated coating CNHH. | ||
Conclusion
In summary, an ordered mesoporous silica coating used for optical AR coating with face-center orthorhombic symmetry structure was successfully prepared using TEOS as silica precursor and F127 as a surfactant. To improve the properties of AR coating, the coating was modificated by various post-treatment. Ammonia-treated ordered mesoporous AR coating showed high transmittance. Ammonia and HMDS treatment decreased the condensation and increased the hydrophobicity. After twice HMDS treatment, the hydrophobicity of AR coating had a greater increase. In addition, ordered pore structure was maintained after various post-treatments. Twice HMDS treated ordered mesoporous silica AR coating is a good candidate for the future application of solar collector covers.Acknowledgements
This work was supported by the National Key Native Science Foundation, China (no. 10835008) and National Science Foundation of Shanxi (no. 2012011005-1). The authors wish to acknowledge 1W2A beamline of Beijing Synchrotron Radiation Facility and BL16B1 beamline of Shanghai Synchrotron Radiation Facility in China. Additionally, the authors gratefully acknowledge the assistance of Prof. Hugh W. Hillhouse and Dr Steve Gaik of University of Washington during the analysis of GISAXS patterns.References
- G. S. Vicente, R. Bayón, N. Germán and A. Morales, Sol. Energy, 2009, 85, 676–680 CrossRef PubMed.
- S. Guldin, P. Kohn, M. Stefik, J. Song, G. Divitini, F. Ecarla, C. Ducati, U. Wiesner and U. Steiner, Nano Lett., 2013, 13, 5329–5335 CrossRef CAS PubMed.
- H. J. Gwon, Y. Park, C. W. Moon, S. Nahm, S.-J. Yoon, S. Y. Kim and H. W. Jang, Nano Res., 2014, 7, 670–678 CrossRef CAS.
- B. E. Yoldas, Appl. Opt., 1980, 19, 1425–1429 CrossRef CAS PubMed.
- J. A. Hiller, J. D. Mendelsohn and M. F. Rubner, Nat. Mater., 2002, 1, 59–63 CrossRef CAS PubMed.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- C.-H. Chen, S.-Y. Li, A. S. T. Chiang, A. T. Wu and Y. S. Sun, Sol. Energy Mater. Sol. Cells, 2011, 95, 1694–1700 CrossRef CAS PubMed.
- H. P. Ye, X. X. Zhang, Y. L. Zhang, L. Q. Ye, B. Xiao, H. B. Lv and B. Jiang, Sol. Energy Mater. Sol. Cells, 2011, 95, 2347–2351 CrossRef CAS PubMed.
- X. G. Li and J. Shen, J. Sol–Gel Sci. Technol., 2011, 59, 539–545 CrossRef CAS PubMed.
- Y. Liu, J. Shen, B. Zhou, G. M. Wu and Z. H. Zhang, J. Sol–Gel Sci. Technol., 2013, 68, 81–87 CrossRef CAS.
- X. X. Zhang, S. Cai, D. You, L. H. Yan, H. B. Lv, X. D. Yuan and B. Jiang, Adv. Funct. Mater., 2013, 23, 4361–4365 CrossRef CAS.
- H. J. Jeong, D. K. Kim, S. B. Lee, S. H. Kwon and K. Kadono, J. Colloid Interface Sci., 2001, 235, 130–134 CrossRef CAS PubMed.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS.
- D. Y. Zhao, P. D. Yang, N. Melosh, J. L. Feng, B. F. Chemelka and G. D. Stucky, Adv. Mater., 1998, 10, 1380–1385 CrossRef CAS.
- J. Bravo, L. Zhai, Z. Wu, R. E. Cohen and M. F. Rubner, Langmuir, 2007, 23, 7293–7298 CrossRef CAS PubMed.
- Y. Du, L. E. Luna, W. S. Tan, M. F. Rubner and R. E. Cohen, ACS Nano, 2010, 4, 4308–4316 CrossRef CAS PubMed.
- J. H. sun, Q. H. Zhang, R. M. Ding, H. B. Lv, H. W. Yan, X. D. Yuan and Y. Xu, Phys. Chem. Chem. Phys., 2014, 16, 16684–16693 RSC.
- R. E. Williford, X. S. Li, R. S. Addleman, G. E. Fryxell, S. Baskaran, J. C. Birnbaum, C. Coyle, T. S. Zemanian, C. Wang and A. R. Courtney, Microporous Mesoporous Mater., 2005, 85, 260–266 CrossRef CAS PubMed.
- X. S. Meng, Y. Wang, H. N. Wang, J. Zhong and R. Y. Chen, Sol. Energy, 2014, 101, 283–290 CrossRef CAS PubMed.
- P. Falcaro, S. Costacurta, G. Mattei, H. Amenitsch, A. Marcelli, M. C. Guidi, M. Piccinini, A. Nucara, L. Malfatti, T. Kidchob and P. Innocenzi, J. Am. Chem. Soc., 2005, 127, 3838–3846 CrossRef CAS PubMed.
- M. Matheron, A. Bourgeois, T. Gacoin, A. Brunet-Bruneau, P.-A. Albouy, J.-P. Boilot, J. Biteau and P. Lacan, Thin Solid Films, 2006, 495, 175–179 CrossRef CAS PubMed.
- D. Grosso, A. R. Balkenende, P. A. Albouy, A. Ayral, H. Amenitsch and F. Babonneau, Chem. Mater., 2001, 13, 1848–1856 CrossRef CAS.
- T.-J. Ha, H.-H. Park, S.-B. Jung, H. Ryu and B.-G. Yu, J. Colloid Interface Sci., 2008, 326, 186–190 CrossRef CAS PubMed.
- D. Grosso, F. Cagnol, G.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J.
J. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) de
de![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A.
A. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A. Soler-Illia, E.
A. Soler-Illia, E. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L. Crepaldi, H. Amenitsch, A. Brunet-Bruneau, A. Bourgeois and C. Sanchez, Adv. Funct. Mater., 2004, 14, 309–322 CrossRef CAS.
L. Crepaldi, H. Amenitsch, A. Brunet-Bruneau, A. Bourgeois and C. Sanchez, Adv. Funct. Mater., 2004, 14, 309–322 CrossRef CAS. - S. Tanaka, Y. Katayama, M. P. Tate, H. W. Hillhouse and Y. Miyake, J. Mater. Chem., 2007, 17, 3639–3645 RSC.
- P. Innocenzi, L. Malfatti, T. Kidchob and P. Falcaro, Chem. Mater., 2009, 21, 2555–2564 CrossRef CAS.
- M. P. Tate, V. N. Urade, J. D. Kowalski, T. C. Wei, B. D. Hamilton, B. W. Eggiman and H. W. Hillhouse, J. Phys. Chem. B, 2006, 110, 9882–9892 CrossRef CAS PubMed.
- T. C. Wei and H. W. Hillhouse, Langmuir, 2007, 23, 5689–5699 CrossRef CAS PubMed.
- S. Tanaka, M. P. Tate, N. Nishiyama, K. Ueyama and H. W. Hillhouse, Chem. Mater., 2006, 18, 5461–5466 CrossRef CAS.
- P. Falcaro, D. Gross, H. Amenitsch and P. Innocenzi, J. Phys. Chem. B, 2004, 108, 10942–10948 CrossRef CAS.
- S. Besson, C. Ricolleau, T. Gacoin, C. Jacquiod and J. P. Boilot, Microporous Mesoporous Mater., 2003, 60, 43–49 CrossRef CAS.
- Y. K. Hwang, K. R. Patil, S. H. Jhung, J. S. Chang, Y. J. Ko and S. E. Park, Microporous Mesoporous Mater., 2005, 78, 245–253 CrossRef CAS PubMed.
- Q. S. Lu, Z. Y. Wang, P. Y. Wang and J. G. Li, Nanoscale Res. Lett., 2010, 5, 761–768 CrossRef CAS PubMed.
- N. Kitazawa, H. Sato and Y. Watanabe, J. Mater. Sci., 2007, 42, 5074–5079 CrossRef CAS PubMed.
- E. K. Richman, T. Brezesinski and S. H. Tolbert, Nat. Mater., 2008, 7, 712–717 CrossRef CAS PubMed.
- M. Bom and E. Wolf, Principles of optics, Pergamon, New York, 1980, vol. 19891, pp. 747–754 Search PubMed.
- C. S. Thompson, R. A. Fleming and M. Zou, Sol. Energy Mater. Sol. Cells, 2013, 115, 108–113 CrossRef CAS PubMed.
- K. Tadanaga, J. Morinaga, A. Matsuda and T. Minami, Chem. Mater., 2000, 12, 590–592 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |






