Preparation of SiO2/dye luminescent nanoparticles and their application in light-converting films
Abstract
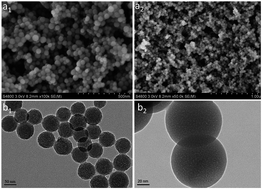
Highly luminescent and photostable dye-h-silica (dye-hybrid-silica) nanoparticles (NPs) were prepared using a base-catalyzed reverse microemulsion method. We obtained dye-h-silica nanoparticles with ∼65 nm radius, a narrow particle size distribution, high fluorescence intensity, and a controlled internal architecture. The luminescence intensity of the dye-h-SiO2 is 2.4 times higher than that of the pure dye in aqueous solution. The incorporation of dye molecules into the silica NPs protects the dye from the surrounding environment, as the silica layer is a good shield against oxygen and moisture, so that the dye-h-silica monoliths (SMs) maintain their initial luminescence after the encapsulation curing process. Green and red light-emitting dye-h-SMs are successfully applied to the preparation of remote trigger LEDs.

 Please wait while we load your content...
Please wait while we load your content...