Rare observation of ‘aggregation induced emission’ in cyclometalated platinum(ii) complexes and their biological activities†
Abstract
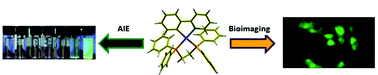
Three strong solid state emissive cyclometalated platinum(II) complexes [Pt(C⁁N) (CH⁁N) (Cl)] (1) (C⁁N/CH⁁N = 2-phenylpyridine, C⁁N = bidentate and CH⁁N = monodentate), [Pt(C⁁N) (P⁁P)]Cl [P⁁P = bis(diphenylphosphino)ethane (2) and cis-1,2-bis(diphenylphosphino)ethene (3)] were reported. These were identified as ‘Aggregation Induced Emission (AIE)’ active complexes based on controlled experiments. Cytotoxicity and cell imaging have been studied for the complex 2.

 Please wait while we load your content...
Please wait while we load your content...