Lipase catalyzed synthesis of 3,3′-(arylmethylene)bis(2-hydroxynaphthalene-1,4-dione)†
Haoran Wang‡
a,
Zhi Wang‡a,
Chunyu Wangb,
Fengjuan Yanga,
Hong Zhanga,
Hong Yuea and
Lei Wang*a
aKey Laboratory of Molecular Enzymology and Engineering of Ministry of Education, College of Life Science, Jilin University, Changchun 130023, P R China. E-mail: w_lei@jlu.edu.cn
bState key Laborarory of Supramolecular Structure and Materials, Jilin University, Changchun 130023, China
First published on 6th August 2014
Abstract
As a green and inexpensive biocatalyst, lipase has been used to catalyze the synthesis of 3,3′-(arylmethylene)bis(2-hydroxynaphthalene-1,4-dione) (3) for the first time. The products could be obtained in excellent yields (82.2–94.8%, 2 h). This study not only provides a simple and efficient method, but also expands the biocatalytic promiscuity of lipase in organic synthesis.
2-Hydroxy-1,4-naphthoquinone (Lawsone), isolated from henna (Lawsonia inermis), has been traditionally used as hair dye, body paint and tattoo dye.1–4 Recently, some researchers have reported that lawsone and its derivatives also have some important biological activities, including antibacterial, anticancer and antioxidant activity.5–7 3,3′-(Arylmethylene)bis(2-hydroxynaphthalene-1,4-dione) (3) is one type of its derivatives. It could be synthesized with 2-hydroxy-1,4-naphthoquinone (1) and aromatic aldehyde (2) as the substrates catalyzed by LiCl or Et3N·HCl.8–10 However, these catalysts suffer from some limitations such as environmentally hazardous catalyst, high reaction temperature or long reaction time. Thus, an environmentally benign and effective catalyst is needed for the synthesis of 3,3′-(arylmethylene)bis(2-hydroxynaphthalene-1,4-dione) (3).
As the “hidden skills” of enzyme, enzyme catalytic promiscuity is the ability of an enzyme of catalyzing more than one type of chemical transformation, and has been exploited in organic synthesis.11,12 Lipase is one of the most used biocatalyst in the area of enzyme catalytic promiscuity due to their broad specificity and excellent stability in organic solvents. Recent reports have indicated that lipase can create carbon–carbon bonds in organic synthesis (Aldol condensation, Morita–Baylis–Hillman reaction, Michael addition, Markovnikov addition, and Knoevenagel reaction, et al.).13–17 As a part of our research to explore the applications of lipase in this new area, we are focusing on the synthesis of 3,3′-(arylmethylene)bis(2-hydroxynaphthalene-1,4-dione) (3) in this study. To the best of our knowledge, it is reported for the first time that the synthesis of 3,3′-(arylmethylene)bis(2-hydroxynaphthalene-1,4-dione) (3) can be catalyzed by lipase (Scheme 1).
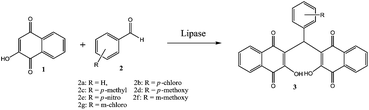 | ||
| Scheme 1 Synthesis of 3,3′-(arylmethylene)bis(2-hydroxynaphthalene-1,4-dione) (3) catalyzed by lipase. | ||
Initially, the synthesis of 3,3′-(phenylmethylene)bis(2-hydroxynaphthalene-1,4-dione) (3a) was selected as a model reaction. It's known that the catalytic activity of enzyme depends mainly on the type and origin of the enzyme.18 In this study, lipases from 6 different sources were selected to catalyze this reaction (Table 1). It could be found that all the selected lipases can catalyze the reaction. Moreover, this enzymatic reaction was very clean and no side product could be detected in this reaction. The highest yield (88.3%) was achieved by using CSL as catalyst. Therefore, CSL was chosen as a catalyst for further study. Compared with the reaction in the absence of enzyme, no obvious difference could be found when the denatured CSL or BSA was selected as catalyst in this reaction. These results suggested that a special active conformation of enzyme should be needed in this reaction.
| Entry | Enzyme | Yieldb (%) |
|---|---|---|
| a Reaction condition: 2-hydroxy-1,4-naphthoquinone (2 mmol), benzaldehyde (1 mmol), ethanol (10 mL), enzyme (30 mg, protein content), 60 °C, 2 h.b Isolated yields.c CSL was denatured by heating it to 100 °C for 6 h in water before lyophilization. | ||
| 1 | Porcine pancreas lipase (PPL) | 77.8 ± 2.6 |
| 2 | Candida antarctica lipase B (CALB) | 56.1 ± 2.1 |
| 3 | Pseudomonas sp. lipase (PSL) | 64.2 ± 3.8 |
| 4 | C. rugosa lipase (CRL) | 52.9 ± 2.1 |
| 5 | Candida sp. lipase (CSL) | 88.3 ± 3.5 |
| 6 | Pseudomonas fluorescens lipase (PFL) | 59.4 ± 3.3 |
| 7 | Bovine serum albumin (BSA) | 22.3 ± 5.2 |
| 8 | Candida sp. lipase (denatured)c | 25.7 ± 4.9 |
| 9 | No enzyme | 19.4 ± 4.7 |
Generally, organic solvent could dramatically influence the enzyme catalytic performance.19 Thus, six organic solvents were screened for the reaction and the results are listed in Table 2. The results clearly demonstrated that the reaction rate was influenced by the solvent. It should be attributed to the conformation change of enzyme in different solvents. According to the results, the highest yield was obtained while ethanol was selected as the reaction media.
| Entry | Solvent | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
Yieldb (%) |
|---|---|---|---|
| a Reaction condition: 2-hydroxy-1,4-naphthoquinone (2 mmol), benzaldehyde (1 mmol), organic solvent (10 mL), CSL (30 mg, protein content), 60 °C, 2 h.b Isolated yields. | |||
| 1 | N,N-Dimethylformamide | −1.04 | 80.4 ± 3.1 |
| 2 | Acetonitrile | −0.33 | 54.4 ± 3.9 |
| 3 | Ethanol | −0.24 | 88.3 ± 3.5 |
| 4 | Tetrahydrofuran | 0.46 | 30.6 ± 4.7 |
| 5 | Dioxane | −0.27 | 42.8 ± 4.6 |
| 6 | Dimethyl sulfoxide | −1.35 | 72.2 ± 3.3 |
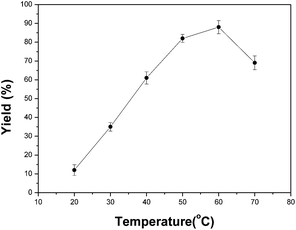
Temperature is another important factor in a lipase-catalyzed reaction.20 In the present study, the reaction temperature was changed from 20 to 70 °C to investigate its effect. The results shown in Fig. 1 indicated that the yield was greatly improved by raising the temperature, and reached the highest yield of 88.3% at 60 °C after 2 h. However, a further increase in the temperature (above 60 °C) may lead to the denaturation of enzyme and thus decrease the reaction rate.§
The effect of the amount of lipase on the yield has also been investigated (data not shown here). It could be found that the use of lower amount of lipase (10 mg or 20 mg) required a longer reaction time (>2 h) to afford a comparable result. By increasing the amount of enzyme, the number of active sites that took part in the reaction would increase.21 Consequently, the reaction rate was increased. However, there was no obvious difference between 30 and 40 mg lipase in 10 mL reaction system. So it was believed that 30 mg lipase was sufficient for the reaction.
To explore the scope and generality of the method, a variety of aromatic aldehydes were selected as the substrates under the optimal conditions. As shown in Table 3, the reaction is applicable to various aromatic aldehydes containing nitro, chlorine, methoxyl and methyl substituent (yields ranging from 82.2% to 94.8%). It was noteworthy that no obvious substituent effect (electron-donating or electron-withdrawing groups) was found in this reaction.
| Entry | Aldehyde | Product | Yieldb (%) |
|---|---|---|---|
| a Reaction condition: 2-hydroxy-1,4-naphthoquinone (2 mmol), aldehyde (1 mmol), ethanol (10 mL), CSL (30 mg, protein content), 60 °C, 2 h.b Isolated yields. | |||
| 1 | 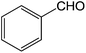 |
3a | 88.3 ± 3.5 |
| 2 | 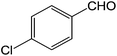 |
3b | 92.1 ± 2.7 |
| 3 | 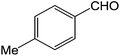 |
3c | 84.6 ± 3.1 |
| 4 | 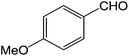 |
3d | 94.8 ± 1.9 |
| 5 | 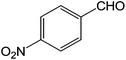 |
3e | 82.2 ± 2.2 |
| 6 | 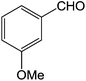 |
3f | 85.1 ± 2.8 |
| 7 | 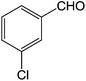 |
3g | 85.5 ± 2.4 |
We attempted to elucidate a reaction pathway of this reaction (Scheme 2). The first step is the deprotonation of 2-hydroxy-1,4-naphthoquinone (1) catalyzed by lipase to form an enolate ion (1′). Secondly, aldehyde (2) accepted the proton and simultaneously connected the enolate ion (1′) to obtain an intermediate (4) with the forming a carbon–carbon bond. Then, the Knoevenagel product (5) could be formed by dehydration. In the final step, the product (5) reacted further with another molecule of 2-hydroxy-1,4-naphthoquinone (1) to afford the corresponding product (3). Many reports have suggested that the promiscuous catalysis reactions should be mediated by a Ser-His-Asp catalytic triad in the active site of lipase.22–24 However, it's not very clear that the catalytic triad take part in this type of reaction. The use of some irreversible inhibitors of the serine hydrolases will be applied to clarify the mechanism and the results will be reported in due course.
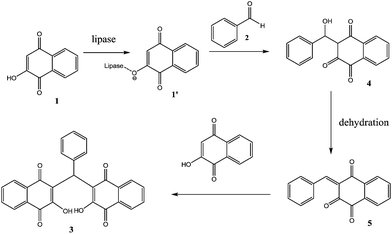 | ||
| Scheme 2 Proposed mechanism of the lipase-catalyzed synthesis of 3,3′-(arylmethylene)bis(2-hydroxynaphthalene-1,4-dione). | ||
In conclusion, we have reported for the first time that lipase can catalyze the synthesis of 3,3′-(arylmethylene)bis(2-hydroxynaphthalene-1,4-dione) with high yields (82.2–94.8%). The influence of reaction conditions including enzyme source, reaction media, temperature and enzyme loading has been investigated. It provides a new case of lipase catalytic promiscuity and widens the application of lipase in organic synthesis. In an organic medium, free lipase is aggregated at considerable degree. Therefore, most of the active sites of enzymes are confined inward. The use of immobilized enzymes can avoid this problem. Furthermore, immobilization may also improve enzyme properties, such as stability, selectivity or specificity.25–30 In order to improve the performance of lipase in this reaction, a study of enzyme immobilization is currently in progress and will be reported in due course.
Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (no. 21172093, 31070708) and the Natural Science Foundation of Jilin Province of China (no. 201115039, 20140101141JC) for the financial support.Notes and references
- M. Liang and J. Chen, Chem. Soc. Rev., 2013, 42, 3488 RSC.
- B. G. Kim, K. Chung and J. Kim, Chem.–Eur. J., 2013, 19, 5220 CrossRef CAS PubMed.
- J. Sangeetha and J. Philip, RSC Adv., 2013, 3, 8047 RSC.
- S. Ali, T. Hussain and R. Nawaz, J. Cleaner Prod., 2009, 17, 61 CrossRef CAS PubMed.
- F. S. Ghaheh, S. M. Mortazavi, F. Alihosseini, A. Fassihi, A. S. Nateri and D. Abedi, J. Cleaner Prod., 2014, 72, 139 CrossRef PubMed.
- J. K. Govind, G. S. Rao, S. Rajagopalan, I. Eiichiro, T. Midori, I. Akira and T. Harukuni, Anti-Cancer Agents Med. Chem., 2013, 13, 1500 CrossRef.
- R. B. Semwal, D. K. Semwal, S. Combrinck, C. Cartwright-Jones and A. Viljoen, J. Ethnopharmacol., 2014, 5, 42 Search PubMed.
- Z. N. Tisseh and A. Bazgir, Dyes Pigm., 2009, 83, 258 CrossRef CAS PubMed.
- C. E. Dalgliesh, J. Am. Chem. Soc., 1949, 71, 1697 CrossRef CAS.
- K. Bock, N. Jacobsen and B. Terem, J. Chem. Soc., Perkin Trans. 1, 1986, 1, 659 RSC.
- I. Nobeli, A. D. Favia and J. M. Thornton, Nat. Biotechnol., 2009, 27, 157 CrossRef CAS PubMed.
- M. Kapoor and M. N. Gupta, Process. Biochem., 2012, 47, 555 CrossRef CAS PubMed.
- M. T. Reetz, R. Mondiere and J. D. Carballeira, Tetrahedron Lett., 2007, 48, 1679 CrossRef CAS PubMed.
- M. Svedendahl, K. Hult and P. Berglund, J. Am. Chem. Soc., 2005, 127, 17988 CrossRef CAS PubMed.
- W. B. Wu, N. Wang, J. M. Xu, Q. Wu and X. F. Lin, Chem. Commun., 2005, 2348 RSC.
- D. F. Izquierdo, O. Barbosa, M. I. Burguete, P. Lozano, S. V. Luis, R. Fernandez-Lafuente and E. García-Verdugo, RSC Adv., 2014, 4, 6219 RSC.
- F. J. Yang, Z. Wang, H. R. Wang, H. Zhang, H. Yue and L. Wang, RSC Adv., 2014, 4, 25633 RSC.
- Z. Wang, R. Wang, J. Tian, B. Zhao, X. F. Wei, Y. L. Su, C. Y. Li, S. G. Cao, T. F. Ji and L. Wang, J. Asian Nat. Prod. Res., 2010, 12, 56 CrossRef CAS PubMed.
- E. N. Xun, X. L. Lv, W. Kang, J. X. Wang, H. Zhang, L. Wang and Z. Wang, Appl. Biochem. Biotechnol., 2012, 168, 697 CrossRef CAS PubMed.
- S. Bai, Z. Guo, W. Liu and Y. Sun, Food Chem., 2006, 96, 1 CrossRef CAS PubMed.
- H. Zhang, Z. Wang, C. Y. Wang, H. R. Wang, T. X. Cheng and L. Wang, RSC Adv., 2014, 4, 19512 RSC.
- S. Jung, J. Kim and S. Park, RSC Adv., 2013, 3, 2590 RSC.
- C. Branneby, P. Carlqvist, A. Magnusson, K. Hult, T. Brinck and P. Berglund, J. Am. Chem. Soc., 2003, 125, 874 CrossRef CAS PubMed.
- K. Świderek, S. Martí and V. Moliner, ACS Catal., 2014, 4, 426 CrossRef.
- R. C. Rodrigues, C. Ortiz, A. Berenguer-Murcia, R. Torres and R. Fernández-Lafuente, Chem. Soc. Rev., 2013, 42, 6290 RSC.
- D. A. Cowan and R. Fernandez-Lafuente, Enzyme Microb. Technol., 2011, 49, 326 CrossRef CAS PubMed.
- R. C. Rodrigues, Á. Berenguer-Murcia and R. Fernandez-Lafuente, Adv. Synth. Catal., 2011, 353, 2216 CrossRef CAS PubMed.
- K. Hernandez and R. Fernandez-Lafuente, Enzyme Microb. Technol., 2011, 48, 107 CrossRef CAS PubMed.
- C. Mateo, J. M. Palomo, G. Fernandez-Lorente, J. M. Guisan and R. Fernandez-Lafuente, Enzyme Microb. Technol., 2007, 40, 1451 CrossRef CAS PubMed.
- V. Stepankova, S. Bidmanova, T. Koudelakova, Z. Prokop, R. Chaloupkova and J. Damborsky, ACS Catal., 2013, 3, 2823 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra06516f |
| ‡ These authors contributed equally to this work. |
| § A typical enzymatic procedure of the reaction: lipase (30 mg) was added to a 25 mL round-bottom flask containing aldehyde (1 mmol), 2-hydroxy-1,4-naphthoquinone (2 mmol) and organic solvent (10 mL). The mixture was maintained at 60 °C and shaken at 200 rpm for 2 h (the reaction was monitored by TLC). After completion of the reaction, the solvent was evaporated, and the residue was washed with water and ethanol to afford the pure product. All the isolated products were well characterized by NMR spectroscopy. |
| This journal is © The Royal Society of Chemistry 2014 |