An expedient total synthesis of mupirocin H†
P. Srihari*a,
N. Hari Krishnaab,
Y. Sridhara,
A. Krishnam Rajub and
Ahmed Kamalbc
aDivision of Natural Products Chemistry, CSIR–Indian Institute of Chemical Technology, Hyderabad-500 007, India. E-mail: srihari@iict.res.in; Fax: +91 4027 1605 12; Tel: +91 4027 1918 15
bDepartment of Medicinal Chemistry, NIPER, Hyderabad-500037, India
cMedicinal Chemistry & Pharmacology Division, CSIR-Indian Institute of Chemical Technology, Hyderabad-500 007, India
First published on 29th July 2014
Abstract
An efficient stereoselective total synthesis of (+)-mupirocin H is described. The chiron and asymmetric strategies were appropriately utilized for the rapid construction of the novel five-membered lactone with six stereogenic centres. The C3–C5 triol segment was derived directly from readily-available D-ribose, and the chirality at the C6 position was introduced by means of substrate-controlled conjugate addition. The remaining chiral centers (C10 and C11) were obtained by Oppolzer's protocol, and the olefin was generated through a Julia–Kocienski olefination.
Introduction
Mupirocin is a mixture of pseudomonic acids containing 95% of pseudomonic acid A along with pseudomonic acids B–D (Fig. 1). Mupirocin is a polyketide antibiotic used in the treatment of skin infections and is a strong inhibitor of Gram-positive bacteria and mycoplasmal pathogens, including methicillin-resistant S. aureus (MRSA).1 Mupirocin W2 (1) and H3 (2), which are often confused with mupirocin, are the pseudomonic acid analogues isolated from Pseudomonas fluorescens. While mupirocin W has been found to display reduced activity compared to P. Fluorescens against Bacillus subtilis,2 the biological activity of mupirocin H, the first truncated analogue of pseudomonic acid, has yet to be investigated. The pseudomonic acid class of molecules all bear a similar C1–C14 carbon framework (Fig. 1) with a central tetrahydropyran ring, whereas mupirocin H and W lack the characteristic tetrahydropyran nucleus and instead contain a 5-membered tetrahydrofuranone/tetrahydrofuran moiety.The unusual structures of the pseudomonic acid class of compounds along with their striking biological properties have provoked many attempts to target them for individual synthesis.4 Chakraborty et al.5a accomplished the first total synthesis of mupirocin H in 19 steps using D-glucose as the chiral source and Julia–Kociensky olefination as the key step. Later, Willis et al.5b demonstrated a convergent synthesis of mupirocin H while developing a strategy for the synthesis of functionalized γ-lactones. While our work was in progress, She et al.5c disclosed a scalable approach involving seven steps (longest linear sequence) for the synthesis of mupirocin H employing Suzuki–Miyaura coupling and Mukaiyama aldol reaction as the key steps. In continuation of our work on the total synthesis of natural products,6 we have recently accomplished the total synthesis of pseudomonic acid methyl monate C.7 Herein, we describe an efficient strategy for the total synthesis of mupirocin H following a chiron approach.
Results and discussion
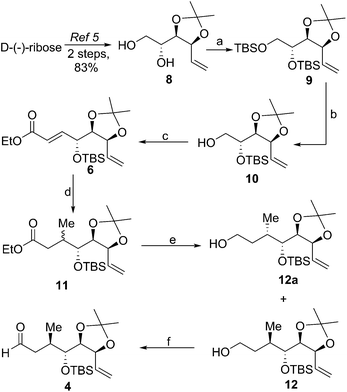
Retrosynthetically, it was envisioned that the target compound mupirocin H could be synthesized from the advanced precursor 3 by the selective oxidation of the terminal olefin followed by functional group interconversion (Scheme 1). The intermediate 3 was planned to be obtained by the coupling of two counterparts, aldehyde 4 and sulfone 5, via a Julia–Kocienski olefination reaction. The aldehyde 4 can be synthesized from α,β-unsaturated ester 6 (in a three-step sequence), and 6 be easily prepared from D-(−)-ribose following established synthetic techniques. Sulfone 5 can be accessed from propionate 7 by following Oppolzer's aldol protocol.With the devised synthetic strategy, we achieved the rapid construction of the Michael acceptor 6 from the known diol 8 (ref. 8) (Scheme 2) obtained from D-(−)-ribose in two steps. The twofold silylation of 8 with tert-butyldimethylsilyl chloride (TBSCl) in the presence of imidazole provided 9, which on mono-desilylation with HF-pyridine afforded the known alcohol 10.9 The Swern oxidation10 of the alcohol 10 followed by Horner Wittig Emmons olefination under the defined conditions for α-oxygenated aldehydes11 gave the ester 6.
The key C6 methyl substituent was installed by taking advantage of the neighbouring chirality in γ-alkoxy-α,β-enoate 6 (Scheme 2), where in the methyl nucleophile was introduced onto the Michael acceptor 6 by the substrate-controlled conjugate addition12 of dimethyl lithium cuprate in the presence of the electrophilic additive TMSCl in a stereo-controlled manner to provide the unseparable diastereomeric mixture of 11 (dr ratio 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The stereoselectivity in this process can be explained by Kornieko's reductive elimination analogy13 and by following the possible transition states (Fig. 2). The diastereomers formed in this stage were separated after reduction of the ester functionality with LiAlH4 to yield the corresponding alcohols 12 and 12a. Based on the theoretical predictions, we proceeded further with the major isomer 12 towards oxidation under Dess–Martin conditions14 to afford the desired aldehyde 4. The stereochemistry of 12 at the C6 carbon was later validated by its utility in synthesizing the target mupirocin H 2 (vide infra).
1). The stereoselectivity in this process can be explained by Kornieko's reductive elimination analogy13 and by following the possible transition states (Fig. 2). The diastereomers formed in this stage were separated after reduction of the ester functionality with LiAlH4 to yield the corresponding alcohols 12 and 12a. Based on the theoretical predictions, we proceeded further with the major isomer 12 towards oxidation under Dess–Martin conditions14 to afford the desired aldehyde 4. The stereochemistry of 12 at the C6 carbon was later validated by its utility in synthesizing the target mupirocin H 2 (vide infra).
 | ||
| Fig. 2 Proposed reductive elimination model-based transition states for the diastereoselective conjugate addition process. | ||
For the synthesis of the sulfone 5, we started with the aldol addition of Oppolzer's propionate 7 on acetaldehyde.15 When the silyl ketene-N,O-acetal of 7 (generated in situ by the treatment of 7 with Et3N and TBSOTf) was added to a solution of acetaldehyde and TiCl4 in CH2Cl2 at −78 °C, the reaction proceeded smoothly to afford the enantiopure aldol adduct 13. Protection of the secondary alcohol as the corresponding silyl ether 14 (TBS ether) followed by cleavage of sultam with LiAlH4 provided the alcohol 15. Alcohol 15 was transformed to a sulfide with phenyl tetrazole thiol under Mitsunobu conditions16 and further oxidized to sulfone 5 using an ammonium molybdate–H2O2 combination without affecting the TBS functionality (Scheme 3).
Coupling of the two fragments aldehyde 4 and sulfone 5 by Julia–Kocienski olefination17 proceeded smoothly under previously optimized conditions7 to provide diene 3 in 80% yield. The selective oxidation of the terminal olefin of diene 3 was again a challenge. In order to get the desired transformation, we first utilized an acetonide-directed regioselective Wacker oxidation18 reaction that quantitatively transformed allylic diol acetonides and carbonates to aldehydes rather than methyl ketones. Our attempts were not successful, however, as the substrate 3 remained inert under the respective conditions. Alternatively, we proceeded with the selective hydroboration of the terminal olefin by boranes. After screening several reagents for this transformation,19 treatment of diene 3 with Cy2BH (ref. 20) followed by oxidation with H2O2 resulted in the successful formation of alcohol 16. Alcohol 16 was further oxidized with a TEMPO–BAIB (ref. 21) combination to deliver the acid precursor 17. We needed to remove the protecting groups and lactonize the acid functionality with C4 alcohol to achieve the target. Towards this, we chose a one-pot cascade process wherein acid 17 was treated with 6 M HCl to facilitate di-TBS deprotection, acetonide deprotection and simultaneous lactonization to give mupirocin H 2 (Scheme 4). The 1H and 13C NMR spectra of this synthetic compound were identical with those of the natural compound3 as well as the previously synthesized mupirocin H.5
Conclusions
In conclusion, we have established a modular and straightforward approach for the synthesis of mupirocin H with an overall yield of 26.8% for the longest linear sequence in eight steps from the known intermediate 10. Further investigation on this synthetic strategy for the synthesis of mupirocin W 1 is currently underway in our laboratory.Experimental section
General methods
1H NMR and 13C NMR spectra were recorded in CDCl3 on a 300- or 500 MHz spectrometer at ambient temperature. The coupling constant J is given in Hz. The chemical shifts are reported as ppm downfield from the TMS internal standard, and signal patterns are indicated as follows: s = singlet, d = doublet, t = triplet, q = quartet, sext = sextet, m = multiplet and br = broad. FTIR spectra were recorded on KBr pellets and are reported in wave number (cm−1). Optical rotations were measured on a digital polarimeter using a 1 mL cell with a path length of 1 dm. For low (MS) and high (HRMS) resolution, m/z ratios are reported as values in atomic mass units. Mass analysis was done in ESI mode. All reagents were reagent grade and used without further purification unless specified otherwise. Solvents for reactions were distilled prior to use; THF and diethyl ether were distilled from Na and benzophenone ketyl, and CH2Cl2 was distilled from CaH2. All air- or moisture-sensitive reactions were conducted under a nitrogen or argon atmosphere in flame- or oven-dried glassware with magnetic stirring. Column chromatography was carried out using silica gel (100–200 mesh) packed in glass columns. Technical grade ethyl acetate and petroleum ether used for column chromatography were distilled prior to use.(R)-5-((4S,5S)-2,2-Dimethyl-5-vinyl-1,3-dioxolan-4-yl)-2,2,3,3,8,8,9,9-octamethyl-4,7-dioxa-3,8-disiladecane (9)
To a magnetically stirring solution of 8 (2.0 g, 10.6 mmol) in CH2Cl2 (20 mL), imidazole (4.34 g, 63.84 mmol), TBSCl (4.8 g, 31.92 mmol), and DMAP (0.64 g, 5.2 mmol) were added at 0 °C under nitrogen atmosphere, and stirring was continued until the starting material was completely consumed (ca. 12 h). The reaction mixture was quenched with sat. aq. NH4Cl (10 mL), diluted with water (10 mL), and extracted with CH2Cl2 (3 × 10 mL). The combined organic layer was washed with brine (10 mL) and dried over anhydrous Na2SO4. Evaporation of CH2Cl2 in vacuo gave the crude product, which was subjected to silica gel column chromatography to give the desired product 9 as a colorless liquid (4.2 g, 95%); Rf = 0.5 (hexane–EtOAc, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]24D = −22.8 (c 0.35, CHCl3); IR (KBr): 2955, 2890, 1642, 1467, 1254, 1093, 835 cm−1; 1H NMR (300 MHz, CDCl3): δ 5.9 (ddd, J = 17.4, 10.4, 7.2 Hz, 1H), 5.33 (d, J = 17.0 Hz, 1H), 5.21 (d, J = 10.4 Hz, 1H), 4.60–4.56 (m, 1H), 4.25–4.21 (m, 1H), 3.81–3.77 (m, 1H), 3.74 (dd, J = 10.8, 3.4 Hz, 1H), 3.67 (dd, J = 10.8, 4.4 Hz, 1H), 1.46 (s, 3H), 1.35 (s, 3H), 0.90 (s, 9H), 0.88 (s, 9H), 0.09 (s, 3H), 0.06 (s, 3H), 0.05 (s, 6H) ppm; 13C NMR (75 MHz, CDCl3): δ 135.1, 117.5, 108.1, 78.8, 77.6, 72.6, 64.8, 27.8, 25.9, 25.4, 18.4, 18.2, −3.8, −4.6, −5.4, −5.5 ppm; MS m/z 439 [M + Na]+; HRMS calcd for C21H44O4NaSi2 [M + Na]+ 439.2670, found 439.2672.
1); [α]24D = −22.8 (c 0.35, CHCl3); IR (KBr): 2955, 2890, 1642, 1467, 1254, 1093, 835 cm−1; 1H NMR (300 MHz, CDCl3): δ 5.9 (ddd, J = 17.4, 10.4, 7.2 Hz, 1H), 5.33 (d, J = 17.0 Hz, 1H), 5.21 (d, J = 10.4 Hz, 1H), 4.60–4.56 (m, 1H), 4.25–4.21 (m, 1H), 3.81–3.77 (m, 1H), 3.74 (dd, J = 10.8, 3.4 Hz, 1H), 3.67 (dd, J = 10.8, 4.4 Hz, 1H), 1.46 (s, 3H), 1.35 (s, 3H), 0.90 (s, 9H), 0.88 (s, 9H), 0.09 (s, 3H), 0.06 (s, 3H), 0.05 (s, 6H) ppm; 13C NMR (75 MHz, CDCl3): δ 135.1, 117.5, 108.1, 78.8, 77.6, 72.6, 64.8, 27.8, 25.9, 25.4, 18.4, 18.2, −3.8, −4.6, −5.4, −5.5 ppm; MS m/z 439 [M + Na]+; HRMS calcd for C21H44O4NaSi2 [M + Na]+ 439.2670, found 439.2672.
(R)-2-((tert-Butyldimethylsilyl)oxy)-2-((4S,5S)-2,2-dimethyl-5-vinyl-1,3-dioxolan-4-yl)ethanol (10)
To the magnetically stirring solution of 9 (7 g, 16.8 mmol) in THF (90 mL) in a Teflon vial, HF–pyridine (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 complex, 4.4 mL) was added at 0 °C, and stirring was continued at the same temperature while the reaction progress was monitored by TLC analysis. After complete consumption of starting material (ca. 12 h), the reaction mixture was neutralized with solid NaHCO3 and filtered. The filtrate was concentrated to give the crude mass, which, upon column purification (EtOAc–hexane 1
3 complex, 4.4 mL) was added at 0 °C, and stirring was continued at the same temperature while the reaction progress was monitored by TLC analysis. After complete consumption of starting material (ca. 12 h), the reaction mixture was neutralized with solid NaHCO3 and filtered. The filtrate was concentrated to give the crude mass, which, upon column purification (EtOAc–hexane 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9), gave the mono TBS ether 10 as a colorless oil (4.6 g, 90%); Rf = 0.5 (hexane–EtOAc, 8
9), gave the mono TBS ether 10 as a colorless oil (4.6 g, 90%); Rf = 0.5 (hexane–EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); [α]24D = −32.8 (c 0.4, CHCl3); IR (KBr): 3339, 2932, 2896, 1466, 1373, 1252, 1079, 836 cm−1; 1H NMR (500 MHz, CDCl3): δ 5.93 (ddd, J = 17.4, 10.3, 7.3 Hz, 1H), 4.91 (d, J = 17.1 Hz, 1H), 5.26 (ddd, J = 10.3, 1.6, 0.9 Hz, 1H), 4.66–4.59 (m, 1H), 4.19 (dd, J = 7.6, 6.2 Hz, 1H), 3.85–3.78 (m, 1H), 3.76–3.68 (m, 2H), 1.48 (s, 3H), 1.38 (s, 3H), 0.88 (s, 9H), 0.10 (s, 3H), 0.07 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 134.1, 118.4, 108.5, 79.5, 78.8, 70.8, 65.0, 27.8, 25.9, 25.4, 18.0, −3.7, −4.4 ppm; MS m/z 325 [M + Na]+; HRMS calcd for C15H30O4NaSi [M + Na]+ 325.1805, found 325.1803.
2); [α]24D = −32.8 (c 0.4, CHCl3); IR (KBr): 3339, 2932, 2896, 1466, 1373, 1252, 1079, 836 cm−1; 1H NMR (500 MHz, CDCl3): δ 5.93 (ddd, J = 17.4, 10.3, 7.3 Hz, 1H), 4.91 (d, J = 17.1 Hz, 1H), 5.26 (ddd, J = 10.3, 1.6, 0.9 Hz, 1H), 4.66–4.59 (m, 1H), 4.19 (dd, J = 7.6, 6.2 Hz, 1H), 3.85–3.78 (m, 1H), 3.76–3.68 (m, 2H), 1.48 (s, 3H), 1.38 (s, 3H), 0.88 (s, 9H), 0.10 (s, 3H), 0.07 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 134.1, 118.4, 108.5, 79.5, 78.8, 70.8, 65.0, 27.8, 25.9, 25.4, 18.0, −3.7, −4.4 ppm; MS m/z 325 [M + Na]+; HRMS calcd for C15H30O4NaSi [M + Na]+ 325.1805, found 325.1803.
(R,E)-Ethyl-4-((tert-butyldimethylsilyl)oxy)-4-((4S,5S)-2,2-dimethyl-5-vinyl-1,3-dioxolan-4-yl)but-2-enoate (6)
Freshly distilled DMSO (3.02 mL, 42.4 mmol) was added dropwise to a cooled (−78 °C) solution of oxalyl chloride (1.83 mL, 21.2 mmol) in anhydrous CH2Cl2 (60 mL). Five minutes later, a solution of alcohol 10 (3.2 g, 10.6 mmoL) in CH2Cl2 (30 mL) was added, and the mixture was stirred at −78 °C for 30 minutes. Subsequently, Et3N (8.9 mL, 63.6 mmol) was added, and stirring continued for 15 minutes at the same temperature and for 30 minutes while slowly warming to room temperature. The reaction was then quenched with 40 mL of phosphate buffer solution and extracted with CH2Cl2 (3 × 20 mL). The combined extracts were washed with water (20 mL) and brine (20 mL), dried over Na2SO4 and concentrated under reduced pressure. The resulting 3.2 g (42.3 mmol, 100%) of pure aldehyde was dissolved in anhydrous DME (20 mL) and added to a mixture of triethyl phosphonoacetate (3.7 g, 15.9 mmol) and n-BuLi (1.6 M in hexane, 9.94 mL) in anhydrous DME (10 mL) that had been pre-stirred for 20 minutes before the addition. Within five minutes, all the starting material was consumed (the reaction was monitored by TLC), and the reaction was quenched with sat. aq. NaHCO3 (10 mL) at 0 °C and extracted with CH2Cl2 (3 × 20 mL). The combined extracts were washed with brine (30 mL), dried over Na2SO4 and concentrated to give the crude product, which, upon silica gel column purification (EtOAc–hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19), gave ester 6 (3.31 g, 85% for two steps) as a pale yellow oil; Rf = 0.5 (hexane–EtOAc, 9
19), gave ester 6 (3.31 g, 85% for two steps) as a pale yellow oil; Rf = 0.5 (hexane–EtOAc, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]24D = +8.0 (c 0.25, CHCl3); IR (KBr): 2935, 2860, 1734, 1466, 1374, 1259, 1077, 840 cm−1; 1H NMR (300 MHz, CDCl3): δ 6.89 (dd, J = 15.7, 6.3 Hz, 1H), 5.97 (dd, J = 15.9, 1.3 Hz, 1H), 6.04–5.93 (m, 1H), 5.38 (d, J = 17.1 Hz, 1H), 5.25 (d, J = 10.3 Hz, 1H), 4.68–4.61 (m, 1H), 4.37–4.30 (m, 1H), 4.20 (q, J = 7.1 Hz, 2H), 4.12–4.06 (m, 1H), 1.46 (s, 3H), 1.34 (s, 3H), 1.29 (t, J = 7.1 Hz, 3H), 0.89 (s, 9H), 0.05 (s, 3H) 0.02 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 166.1, 147.5, 133.8, 122.6, 118.2, 108.7, 80.6, 78.6, 71.2, 60.4, 27.4, 25.8, 25.2, 18.1, 14.2,−3.7, −4.7 ppm; MS m/z 393 [M + Na]+; HRMS calcd for C19H34O5NaSi [M + Na]+ 393.2067, found 393.2067.
1); [α]24D = +8.0 (c 0.25, CHCl3); IR (KBr): 2935, 2860, 1734, 1466, 1374, 1259, 1077, 840 cm−1; 1H NMR (300 MHz, CDCl3): δ 6.89 (dd, J = 15.7, 6.3 Hz, 1H), 5.97 (dd, J = 15.9, 1.3 Hz, 1H), 6.04–5.93 (m, 1H), 5.38 (d, J = 17.1 Hz, 1H), 5.25 (d, J = 10.3 Hz, 1H), 4.68–4.61 (m, 1H), 4.37–4.30 (m, 1H), 4.20 (q, J = 7.1 Hz, 2H), 4.12–4.06 (m, 1H), 1.46 (s, 3H), 1.34 (s, 3H), 1.29 (t, J = 7.1 Hz, 3H), 0.89 (s, 9H), 0.05 (s, 3H) 0.02 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 166.1, 147.5, 133.8, 122.6, 118.2, 108.7, 80.6, 78.6, 71.2, 60.4, 27.4, 25.8, 25.2, 18.1, 14.2,−3.7, −4.7 ppm; MS m/z 393 [M + Na]+; HRMS calcd for C19H34O5NaSi [M + Na]+ 393.2067, found 393.2067.
(3R,4R)-Ethyl-4-((tert-butyldimethylsilyl)oxy)-4-((4S,5S)-2,2-dimethyl-5-vinyl-1,3-dioxolan-4-yl)-3-methylbutanoate (11)
MeLi (1.6 M in hexane, 60.8 mL, 97.3 mmol) was added to a stirring suspension of CuI (9.3 g, 48.65 mmol) in THF (50 mL) at −20 °C. The resulting solution was stirred at this temperature for 20 minutes and then cooled to −78 °C. To this solution, TMSCl (18.6 mL, 145.95 mmol) was added dropwise followed by canulation with a solution of α,β-unsaturated ester 6 (3.0 g, 8.11 mmol) in THF (30 mL) at −78 °C. Within five minutes, all starting material was consumed (reaction monitored by TLC), and the reaction was quenched with NH4OH–NH4Cl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution (100 mL) at −78 °C and allowed to warm to room temperature. The reaction mixture was extracted with EtOAc (3 × 20 mL). The combined extracts were washed with NH4OH–NH4Cl (1
1) solution (100 mL) at −78 °C and allowed to warm to room temperature. The reaction mixture was extracted with EtOAc (3 × 20 mL). The combined extracts were washed with NH4OH–NH4Cl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution (100 mL) and brine (20 mL) solution, dried over anhydrous Na2SO4 and concentrated. The concentrated residue was purified by silica gel column chromatography (EtOAc–hexane 1
1) solution (100 mL) and brine (20 mL) solution, dried over anhydrous Na2SO4 and concentrated. The concentrated residue was purified by silica gel column chromatography (EtOAc–hexane 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19) to afford a diastereomeric mixture of 11 (2.96 g, 95%; 13
19) to afford a diastereomeric mixture of 11 (2.96 g, 95%; 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio as observed in 1H NMR) as a colorless oil; Rf = 0.55 (hexane–EtOAc, 9
1 ratio as observed in 1H NMR) as a colorless oil; Rf = 0.55 (hexane–EtOAc, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); IR (KBr): 2957, 2900, 1736, 1466, 1375, 1253, 1084, 839 cm−1; 1H NMR (500 MHz, CDCl3): δ 5.90 (ddd, J = 17.9, 10.2, 7.6 Hz, 1H), 5.31 (ddd, J = 17.2, 1.7, 1.1 Hz, 1H), 5.23 (d, J = 10.2 Hz, 1H), 4.55–4.50 (m, 1H), 4.18–4.07 (m, 3H), 3.76 (dd, J = 7.9, 2.7 Hz 1H), 2.61 (dd, J = 15.3, 5.2 Hz, 1H), 2.34–2.26 (m, 1H), 2.25 (dd, J = 15.3, 8.7 Hz, 1H), 1.45 (s, 3H), 1.34 (s, 3H), 1.25 (t, J = 7.2 Hz, 3H), 1.02 (d, J = 6.7 Hz, 3H), 0.87 (s, 9H), 0.09 (s, 3H), 0.04 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 173.6, 134.9, 118.4, 108.3, 79.4, 78.8, 72.6, 60.1, 37.7, 35.2, 28.0, 26.0, 25.5, 18.2, 14.4, 14.3, −3.7, −4.3 ppm; MS m/z 409 [M + Na]+; HRMS calcd for C20H38O5NaSi [M + Na]+ 403.2380, found 409.2380.
1); IR (KBr): 2957, 2900, 1736, 1466, 1375, 1253, 1084, 839 cm−1; 1H NMR (500 MHz, CDCl3): δ 5.90 (ddd, J = 17.9, 10.2, 7.6 Hz, 1H), 5.31 (ddd, J = 17.2, 1.7, 1.1 Hz, 1H), 5.23 (d, J = 10.2 Hz, 1H), 4.55–4.50 (m, 1H), 4.18–4.07 (m, 3H), 3.76 (dd, J = 7.9, 2.7 Hz 1H), 2.61 (dd, J = 15.3, 5.2 Hz, 1H), 2.34–2.26 (m, 1H), 2.25 (dd, J = 15.3, 8.7 Hz, 1H), 1.45 (s, 3H), 1.34 (s, 3H), 1.25 (t, J = 7.2 Hz, 3H), 1.02 (d, J = 6.7 Hz, 3H), 0.87 (s, 9H), 0.09 (s, 3H), 0.04 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 173.6, 134.9, 118.4, 108.3, 79.4, 78.8, 72.6, 60.1, 37.7, 35.2, 28.0, 26.0, 25.5, 18.2, 14.4, 14.3, −3.7, −4.3 ppm; MS m/z 409 [M + Na]+; HRMS calcd for C20H38O5NaSi [M + Na]+ 403.2380, found 409.2380.
(3R,4R)-4-((tert-Butyldimethylsilyl)oxy)-4-((4S,5S)-2,2-dimethyl-5-vinyl-1,3-dioxolan-4-yl)-3-methylbutan-1-ol (12)
Ester 11 (3.00 g, 7.76 mmol) was added to a magnetically stirring suspension of LiAlH4 (0.3 g, 7.76 mmol) in THF (20 mL) at 0 °C. Within five minutes, all the starting material was consumed, and the reaction mixture was then quenched with sat. aq. Na2SO4 (5 mL). Silica gel (5.00 g) was added, and the resulting white suspension was stirred at rt for 30 minutes and filtered. The filtrate was dried over anhydrous Na2SO4 and concentrated under reduced pressure to give the crude product. Upon column purification through a short pad of silica gel (hexane–EtOAc 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), alcohol 12 (2.34 g, 88%) was obtained as a colorless oil along with 12a as colorless oil (0.186 g, 7%); Rf = 0.50 (hexane–EtOAc 3
1), alcohol 12 (2.34 g, 88%) was obtained as a colorless oil along with 12a as colorless oil (0.186 g, 7%); Rf = 0.50 (hexane–EtOAc 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); [α]24D = −48.3 (c 0.25, CHCl3); IR (KBr): 3344, 2935, 2860, 1469, 1374, 1253, 1083, 840 cm−1; 1H NMR (500 MHz, CDCl3): δ 5.99–5.87 (m, 1H), 5.36–5.30 (m, 1H), 5.28–5.24 (m, 1H), 4.56–4.51 (m, 1H), 4.14 (dd, J = 7.9, 5.5 Hz, 1H), 3.82 (dd, J = 7.9, 2.5 Hz, 1H), 3.77–3.69 (m, 1H), 3.65–3.59 (m, 1H), 2.01–1.90 (m, 1H), 1.85–1.66 (m, 2H), 1.61–1.52 (m, 1H), 1.45 (s, 3H), 1.35 (s, 3H),1.01 (d, J = 7.0 Hz, 3H), 0.87 (s, 9H), 0.07 (s, 3H) 0.04 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 134.8, 118.5, 108.1, 79.3, 78.9, 73.3, 61.3, 35.1, 34.8, 28.2, 26.0, 25.5, 18.2, 15.3, −3.7, −4.2 ppm; MS m/z 367 [M + Na]+; HRMS calcd for C18H36O4NaSi [M + Na]+ 367.2275, found 367.2279.
2); [α]24D = −48.3 (c 0.25, CHCl3); IR (KBr): 3344, 2935, 2860, 1469, 1374, 1253, 1083, 840 cm−1; 1H NMR (500 MHz, CDCl3): δ 5.99–5.87 (m, 1H), 5.36–5.30 (m, 1H), 5.28–5.24 (m, 1H), 4.56–4.51 (m, 1H), 4.14 (dd, J = 7.9, 5.5 Hz, 1H), 3.82 (dd, J = 7.9, 2.5 Hz, 1H), 3.77–3.69 (m, 1H), 3.65–3.59 (m, 1H), 2.01–1.90 (m, 1H), 1.85–1.66 (m, 2H), 1.61–1.52 (m, 1H), 1.45 (s, 3H), 1.35 (s, 3H),1.01 (d, J = 7.0 Hz, 3H), 0.87 (s, 9H), 0.07 (s, 3H) 0.04 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 134.8, 118.5, 108.1, 79.3, 78.9, 73.3, 61.3, 35.1, 34.8, 28.2, 26.0, 25.5, 18.2, 15.3, −3.7, −4.2 ppm; MS m/z 367 [M + Na]+; HRMS calcd for C18H36O4NaSi [M + Na]+ 367.2275, found 367.2279.
(3R,4R)-4-(tert-Butyldimethylsilyloxy)-4-((4S,5S)-2,2-dimethyl-5-vinyl-1,3-dioxolan-4-yl)-3-methylbutanal (4)
To a magnetically stirring solution of alcohol 12 (1.5 g, 4.35 mmol) in CH2Cl2 (20 mL), Dess–Martin periodinane (2.19 g, 5.22 mmol) was added at 0 °C. Stirring was continued until the starting material was completely consumed (ca. 2 h). The reaction mixture was filtered through a small pad of celite, and the filtrate was washed with sat. aq. NaHCO3 (20 mL), dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the crude product. Upon column purification (hexane–EtOAc, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the aldehyde 4 (1.35 g, 90%) was obtained as a colorless oil. The aldehyde was immediately utilized for the next Julia–Kocienski olefination reaction. Rf = 0.50 (hexane–EtOAc, 4
1), the aldehyde 4 (1.35 g, 90%) was obtained as a colorless oil. The aldehyde was immediately utilized for the next Julia–Kocienski olefination reaction. Rf = 0.50 (hexane–EtOAc, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]24D = −33.5 (c 0.38, CHCl3); IR (KBr): 3448, 2931, 2858, 1726, 1465, 1374, 1253, 1082, 838 cm−1; 1H NMR (500 MHz, CDCl3): δ 9.77 (s, 1H), 5.89 (ddd, 17.4, 9.9, 7.8 Hz, 1H), 5.33 (d, J = 17.2 Hz, 1H), 5.26 (d, J = 10.2 Hz, 1H), 4.54 (t, J = 6.7 Hz, 1H), 4.10 (t, J = 7.2 Hz, 1H), 3.75 (dd, J = 7.2, 1.8 Hz, 1H), 2.80–2.72 (m, 1H), 2.45–2.40 (m, 1H), 2.38 (dd, J = 8.1, 1.5 Hz, 1H), 1.45 (s, 3H), 1.34 (s, 3H), 1.04 (d, J = 6.6 Hz, 3H), 0.89 (s, 9H), 0.10 (s, 3H), 0.06 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 202.6, 134.7, 118.6, 108.3, 79.2, 79.1, 73.0, 47.2, 32.3, 28.0, 26.0, 25.4, 18.2, 15.3, −3.7, −4.2 ppm; MS m/z 365, [M + Na]+; HRMS calcd for C18H34O4NaSi [M + Na]+ 365.2119, found 365.2117.
1); [α]24D = −33.5 (c 0.38, CHCl3); IR (KBr): 3448, 2931, 2858, 1726, 1465, 1374, 1253, 1082, 838 cm−1; 1H NMR (500 MHz, CDCl3): δ 9.77 (s, 1H), 5.89 (ddd, 17.4, 9.9, 7.8 Hz, 1H), 5.33 (d, J = 17.2 Hz, 1H), 5.26 (d, J = 10.2 Hz, 1H), 4.54 (t, J = 6.7 Hz, 1H), 4.10 (t, J = 7.2 Hz, 1H), 3.75 (dd, J = 7.2, 1.8 Hz, 1H), 2.80–2.72 (m, 1H), 2.45–2.40 (m, 1H), 2.38 (dd, J = 8.1, 1.5 Hz, 1H), 1.45 (s, 3H), 1.34 (s, 3H), 1.04 (d, J = 6.6 Hz, 3H), 0.89 (s, 9H), 0.10 (s, 3H), 0.06 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 202.6, 134.7, 118.6, 108.3, 79.2, 79.1, 73.0, 47.2, 32.3, 28.0, 26.0, 25.4, 18.2, 15.3, −3.7, −4.2 ppm; MS m/z 365, [M + Na]+; HRMS calcd for C18H34O4NaSi [M + Na]+ 365.2119, found 365.2117.
(2R,3R)-1-((6S,7aS)-8,8-Dimethyl-2,2-dioxidohexahydro-1H-3a,6-methanobenzo[c]isothiazol-1-yl)-3-hydroxy-2-methylbutan-1-one (13)
To a magnetically stirring solution of propionate 7 (5.00 g, 18.45 mmol) in CH2Cl2 (40 mL), triethylamine (2.82 mL, 20.30 mmol) followed by tert-butyldimethylsilyl-O-triflate (4.66 mL, 20.30 mL) were added at rt. Stirring was continued for 24 h, and the resulting solution was then added to a solution of acetaldehyde (1.14 mL, 20.30 mmol) and TiCl4 (2.23 mL, 20.30 mmol) in CH2Cl2 (30 mL) at −78 °C. After 5 min, the reaction was quenched with sat. aq. NH4Cl (20 mL), diluted with water (50 mL), and stirred at rt for 15 min. The organic layer was separated, and the aqueous layer was extracted with CH2Cl2 (2 × 20 mL). The combined organic layers were then washed with brine (20 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to give the crude product. Upon column purification (hexane–EtOAc, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the crude product gave unreacted propionate 7 (2.00 g) and finally alcohol 13 (3.42 g, 98% brsm) as a white solid (mp = 119–120 °C); Rf = 0.45 (hexane–EtOAc, 7
1), the crude product gave unreacted propionate 7 (2.00 g) and finally alcohol 13 (3.42 g, 98% brsm) as a white solid (mp = 119–120 °C); Rf = 0.45 (hexane–EtOAc, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); [α]24D = −46.17 (c 1.2, CHCl3); IR (KBr): 3526, 2959, 1698, 1683, 1307, 1216, 1062 cm−1; 1H NMR (CDCl3, 300 MHz): δ 3.90 (dd, J = 7.6, 5.1 Hz, 1H), 3.88–3.79 (m, 1H), 3.54 (d, J = 13.9 Hz, 1H), 3.45 (d, J = 13.9 Hz, 1H), 3.16–3.07 (m, 1H), 2.40–2.24 (m, 1H), 2.22–2.03 (m, 2H), 1.98–1.81 (m, 2H), 1.47–1.32 (m, 2H), 1.25 (d, J = 6.2 Hz, 3H), 1.21 (d, J = 6.6 Hz, 3H), 1.18 (s, 3H), 0.97 (s, 3H) ppm; 13C NMR (CDCl3, 75 MHz): δ 175.1, 71.5, 65.3, 53.1, 48.2, 47.6, 46.9, 44.6, 38.4, 32.8, 26.3, 21.8, 20.7, 19.8, 14.0 ppm; MS m/z 316 [M + H]+; HRMS (ESI) for C15H26O4NS [M + H]+ found 316.15682, calcd 316.15771.
3); [α]24D = −46.17 (c 1.2, CHCl3); IR (KBr): 3526, 2959, 1698, 1683, 1307, 1216, 1062 cm−1; 1H NMR (CDCl3, 300 MHz): δ 3.90 (dd, J = 7.6, 5.1 Hz, 1H), 3.88–3.79 (m, 1H), 3.54 (d, J = 13.9 Hz, 1H), 3.45 (d, J = 13.9 Hz, 1H), 3.16–3.07 (m, 1H), 2.40–2.24 (m, 1H), 2.22–2.03 (m, 2H), 1.98–1.81 (m, 2H), 1.47–1.32 (m, 2H), 1.25 (d, J = 6.2 Hz, 3H), 1.21 (d, J = 6.6 Hz, 3H), 1.18 (s, 3H), 0.97 (s, 3H) ppm; 13C NMR (CDCl3, 75 MHz): δ 175.1, 71.5, 65.3, 53.1, 48.2, 47.6, 46.9, 44.6, 38.4, 32.8, 26.3, 21.8, 20.7, 19.8, 14.0 ppm; MS m/z 316 [M + H]+; HRMS (ESI) for C15H26O4NS [M + H]+ found 316.15682, calcd 316.15771.
(2R,3R)-3-((tert-Butyldimethylsilyl)oxy)-1-((6S,7aS)-8,8-dimethyl-2,2-dioxidohexahydro-1H-3a,6-methanobenzo[c]isothiazol-1-yl)-2-methylbutan-1-one (14)
To the stirring solution of alcohol 13 (4.00 g, 12.70 mmol) in CH2Cl2 (40 mL), lutidine (1.6 mL, 14.0 mmol) followed by TBSOTf (3.2 mL, 14.0 mmol) were added at 0 °C. Within 5 min, all the starting material was consumed. Sat. aq. NH4Cl (10 mL) was added, and the reaction was diluted with water (30 mL). The organic layer was separated, and the aqueous layer was extracted with CH2Cl2 (2 × 20 mL). The combined organic layer was washed with brine (20 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to give the crude product. Upon column purification (hexane–EtOAc, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), TBS ether 14 (4.9 g, 90%) was obtained as a white solid (mp = 129 °C); Rf = 0.5 (hexane–EtOAc 7
1), TBS ether 14 (4.9 g, 90%) was obtained as a white solid (mp = 129 °C); Rf = 0.5 (hexane–EtOAc 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); [α]24D = −8.0 (c 0.15, CHCl3); IR (KBr): 2958, 2884, 1693, 1458, 1327, 1220, 1111, 837, 775 cm−1; 1H NMR (500 MHz, CDCl3): δ 4.1–4.13 (m, 1H), 3.89–3.85 (m, 1H), 3.49 (d, J = 13.7 Hz, 1H), 3.41 (d, J = 13.7 Hz, 1H), 3.18–3.13 (m, 1H), 2.08–2.03 (m, 2H), 1.94–1.82 (m, 3H), 1.43–1.30 (m, 2H), 1.16 (s, 3H), 1.11 (d, J = 6.9 Hz, 3H), 1.10 (d, J = 6.3 Hz, 3H), 0.96 (s, 3H), 0.85 (s, 9H), 0.05 (s, 3H), 0.03 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 174.4, 69.9, 65.4, 53.1, 48.0, 47.7, 44.7, 38.6, 32.8, 26.4, 25.7, 20.9, 20.0, 19.8, 17.9, 11.8, −4.6, −4.9 ppm; MS m/z 430 [M + H]+; HRMS calcd for C21H40O4NSSi [M + H]+ 430.2441, found 430.2433.
3); [α]24D = −8.0 (c 0.15, CHCl3); IR (KBr): 2958, 2884, 1693, 1458, 1327, 1220, 1111, 837, 775 cm−1; 1H NMR (500 MHz, CDCl3): δ 4.1–4.13 (m, 1H), 3.89–3.85 (m, 1H), 3.49 (d, J = 13.7 Hz, 1H), 3.41 (d, J = 13.7 Hz, 1H), 3.18–3.13 (m, 1H), 2.08–2.03 (m, 2H), 1.94–1.82 (m, 3H), 1.43–1.30 (m, 2H), 1.16 (s, 3H), 1.11 (d, J = 6.9 Hz, 3H), 1.10 (d, J = 6.3 Hz, 3H), 0.96 (s, 3H), 0.85 (s, 9H), 0.05 (s, 3H), 0.03 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 174.4, 69.9, 65.4, 53.1, 48.0, 47.7, 44.7, 38.6, 32.8, 26.4, 25.7, 20.9, 20.0, 19.8, 17.9, 11.8, −4.6, −4.9 ppm; MS m/z 430 [M + H]+; HRMS calcd for C21H40O4NSSi [M + H]+ 430.2441, found 430.2433.
(2S,3R)-3-((tert-Butyldimethylsilyl)oxy)-2-methylbutan-1-ol (15)
To the magnetically stirring suspension of LiAlH4 (0.177 g, 4.66) in THF (20 mL), amide 14 (2.0 g, 4.66 mmol) was added at 0 °C. Within 5 min, all the starting material was consumed, and the reaction was quenched with sat. aq. Na2SO4 (5 mL). Silica gel (5.00 g) was added, and the resulting white suspension was further stirred at rt for 30 min and filtered. The filtrate was concentrated under reduced pressure to give the crude product. Upon column purification through a short pad of silica gel (hexane–EtOAc 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the crude product gave free sultum auxiliary (0.97 g, 98%) and alcohol 15 (0.92 g, 90%) as a colorless oil; Rf = 0.4 (hexane–EtOAc, 7
1), the crude product gave free sultum auxiliary (0.97 g, 98%) and alcohol 15 (0.92 g, 90%) as a colorless oil; Rf = 0.4 (hexane–EtOAc, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); [α]24D = +19.3 (c 0.2, CHCl3); IR (KBr): 3420, 2957, 2858, 1468, 1377, 1254, 1033, 836, 775 cm_1; 1H NMR (500 MHz, CDCl3): δ 3.85–3.73 (m, 2H), 3.54 (dd, J = 11.0, 5.9 Hz, 1H), 2.95 (br s, 1H), 1.66–1.52 (m, 1H), 1.21 (d, J = 6.2 Hz, 3H), 0.97 (d, J = 7.0 Hz, 3H), 0.89 (s, 9H), 0.10–0.08 (m, 6H) ppm; 13C NMR (75 MHz, CDCl3): δ 73.9, 65.7, 41.6, 25.7, 22.0, 17.8, 14.5, −4.3, −5.1 ppm; MS m/z 219 [M + H]+; HRMS calcd for C11H27O2Si [M + H]+ 219.1774, found 219.1775.
3); [α]24D = +19.3 (c 0.2, CHCl3); IR (KBr): 3420, 2957, 2858, 1468, 1377, 1254, 1033, 836, 775 cm_1; 1H NMR (500 MHz, CDCl3): δ 3.85–3.73 (m, 2H), 3.54 (dd, J = 11.0, 5.9 Hz, 1H), 2.95 (br s, 1H), 1.66–1.52 (m, 1H), 1.21 (d, J = 6.2 Hz, 3H), 0.97 (d, J = 7.0 Hz, 3H), 0.89 (s, 9H), 0.10–0.08 (m, 6H) ppm; 13C NMR (75 MHz, CDCl3): δ 73.9, 65.7, 41.6, 25.7, 22.0, 17.8, 14.5, −4.3, −5.1 ppm; MS m/z 219 [M + H]+; HRMS calcd for C11H27O2Si [M + H]+ 219.1774, found 219.1775.
5-(((2S,3S)-3-((tert-Butyldimethylsilyl)oxy)-2-methylbutyl)sulfonyl)-1-phenyl-1H-tetrazole (5)
PPh3 (0.90 g, 3.44 mmol) and 1-phenyl-1H-tetrazole-5-thiol (0.61 g, 3.44 mmol) were added to a magnetically stirring solution of alcohol 15 (0.50 g, 2.29 mmol) in THF (20 mL) at 0 °C. Finally, diisopropylazodicarboxylate (0.70 mL, 3.44 mmol) was added at the same temperature. Within 30 min, all the starting material was consumed. THF was evaporated under reduced pressure, and the crude solid was purified by column chromatography (hexane–EtOAc 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the sulfide 5a (0.82 g, 95%) as a colorless oil; Rf = 0.5 (hexane–EtOAc, 5
1) to give the sulfide 5a (0.82 g, 95%) as a colorless oil; Rf = 0.5 (hexane–EtOAc, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]24D = +19.9 (c 0.425, CHCl3); IR (KBr): 2957, 2857, 1500, 1408, 1384, 1252, 1082, 835, 773 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.62–7.51 (m, 5H), 3.82–3.76 (m, 1H), 3.65 (dd, J = 12.7, 4.6 Hz, 1H), 3.26 (dd, J = 12.8, 8.1 Hz, 1H), 2.02–1.93 (m, 1H), 1.18 (d, J = 6.3 Hz, 3H), 1.04 (d, J = 6.9 Hz, 3H), 0.88 (s, 9H), 0.05 (s, 3H), 0.03 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 154.9, 133.7, 129.9, 129.7, 123.8, 71.2, 40.1, 36.1, 25.7, 20.8, 17.9, 15.7, −4.3, −5.0 ppm; MS m/z 379 [M + H]+; HRMS calcd for C18H31ON4SSi [M + H]+ 379.1982, found 379.1985.
1); [α]24D = +19.9 (c 0.425, CHCl3); IR (KBr): 2957, 2857, 1500, 1408, 1384, 1252, 1082, 835, 773 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.62–7.51 (m, 5H), 3.82–3.76 (m, 1H), 3.65 (dd, J = 12.7, 4.6 Hz, 1H), 3.26 (dd, J = 12.8, 8.1 Hz, 1H), 2.02–1.93 (m, 1H), 1.18 (d, J = 6.3 Hz, 3H), 1.04 (d, J = 6.9 Hz, 3H), 0.88 (s, 9H), 0.05 (s, 3H), 0.03 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 154.9, 133.7, 129.9, 129.7, 123.8, 71.2, 40.1, 36.1, 25.7, 20.8, 17.9, 15.7, −4.3, −5.0 ppm; MS m/z 379 [M + H]+; HRMS calcd for C18H31ON4SSi [M + H]+ 379.1982, found 379.1985.
To the magnetically stirring solution of sulfide 5a (1.0 g, 2.6 mmol) in EtOH (10 mL), a pre-cooled (0 °C) solution of ammonium molybdate tetrahydrate (1.6 g, 1.4 mmol) in 30% H2O2 (4 mL) was added at 0 °C. Stirring was continued until the starting material was completely consumed (ca. 12 h). Sat. aq. NaCl (10 mL) was added, and the organic layer was extracted with CH2Cl2 (4 × 10 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to give the crude product. Upon column purification (hexane–EtOAc, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), sulfone 5 (0.89 g, 90%) was obtained as a colorless oil; Rf = 0.45 (hexane–EtOAc, 5
1), sulfone 5 (0.89 g, 90%) was obtained as a colorless oil; Rf = 0.45 (hexane–EtOAc, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]24D = +10.1 (c 0.5, CHCl3); IR (KBr): 2929, 2857, 1638, 1497, 1343, 1384, 1254, 1154, 837, 770 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.71–7.66 (m, 2H), 7.65–7.58 (m, 3H), 4.08 (dd, J = 14.8, 2.9 Hz, 1H), 3.83–3.77 (m, 1H), 3.45 (dd, J = 14.6, 9.5 Hz, 1H), 2.33–2.24 (m, 1H), 1.18 (d, J = 6.9 Hz, 3H), 1.18 (d, J = 6.3 Hz, 3H), 0.89 (s, 9H), 0.07 (s, 3H), 0.06 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 154.0, 133.1, 131.4, 129.6, 125.1, 71.4, 57.9, 35.3, 25.8, 21.0, 17.9, 17.1, −4.3, −5.0 ppm; MS m/z 412 [M + H]+; HRMS calcd for C18H31O3N4SSi [M + H]+ 412.1904, found 412.1906.
1); [α]24D = +10.1 (c 0.5, CHCl3); IR (KBr): 2929, 2857, 1638, 1497, 1343, 1384, 1254, 1154, 837, 770 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.71–7.66 (m, 2H), 7.65–7.58 (m, 3H), 4.08 (dd, J = 14.8, 2.9 Hz, 1H), 3.83–3.77 (m, 1H), 3.45 (dd, J = 14.6, 9.5 Hz, 1H), 2.33–2.24 (m, 1H), 1.18 (d, J = 6.9 Hz, 3H), 1.18 (d, J = 6.3 Hz, 3H), 0.89 (s, 9H), 0.07 (s, 3H), 0.06 (s, 3H) ppm; 13C NMR (75 MHz, CDCl3): δ 154.0, 133.1, 131.4, 129.6, 125.1, 71.4, 57.9, 35.3, 25.8, 21.0, 17.9, 17.1, −4.3, −5.0 ppm; MS m/z 412 [M + H]+; HRMS calcd for C18H31O3N4SSi [M + H]+ 412.1904, found 412.1906.
(5S,6R,10R,11R,E)-11-((4S,5S)-2,2-Dimethyl-5-vinyl-1,3-dioxolan-4-yl)-2,2,3,3,5,6,10,13,13,14,14-undecamethyl-4,12-dioxa-3,13-disilapentadec-7-ene (3)
KHMDS (9.73 mL, 0.5 M in toluene, 4.87 mmol) was added dropwise to a stirring solution of sulfone 5 (1.0 g, 2.43 mmol) in dry THF (10 mL) at −78 °C. The resulting bright yellow solution was stirred at −78 °C for 1 h, and a solution of aldehyde 4 (0.84 g, 2.43 mmol) in dry THF (5 mL) was then added slowly from a syringe over 10 min. The reaction mixture was stirred at −78 °C for 1 h and then allowed to warm to rt over 1 h; all starting materials were consumed (monitored by TLC analysis) during this process. The reaction mixture was stirred in open air for 30 min, and all volatiles were then removed under reduced pressure. The obtained residue was purified by column chromatography (2% EtOAc–hexane) to give 3 as a colorless viscous oil (1.03 g, 80%); Rf = 0.3 (5% EtOAc–hexane); [α]24D = −23.18 (c 1.4, CHCl3); IR (KBr): 2958, 2931, 2858, 1466, 1373, 1253, 1081, 837 cm−1; 1H NMR (500 MHz, CDCl3): δ 5.99–5.89 (m, 1H), 5.41–5.31 (m, 2H), 5.31–5.257 (m, 1H), 5.21–5.19 (m, 1H), 4.53–4.47 (m, 1H), 4.12 (dd, J = 7.8, 5.6 Hz, 1H), 3.82 (dd, J = 7.9, 3.1 Hz, 1H), 3.71–3.66 (m, 1H), 2.33–2.26 (m, 1H), 2.16–2.09 (m, 1H), 1.95–1.87 (m, 1H), 1.80–1.72 (m, 1H), 1.46 (s, 3H), 1.34 (s, 3H), 1.03 (d, J = 6.1 Hz, 3H), 0.97 (d, J = 1.6 Hz, 3H), 0.96 (d, J = 1.6 Hz, 3H), 0.88 (s, 9H), 0.87 (s, 9H), 0.06–0.01 (m, 12H) ppm; 13C NMR (75 MHz, CDCl3): δ 135.4, 135.3, 129.3, 117.9, 108.0, 79.5, 78.8, 72.5, 72.0, 44.4, 39.0, 36.1, 28.2, 26.1, 25.9, 20.5, 18.3, 18.1, 16.1, 14.5, −3.7, −4.0, −4.4, −4.8 ppm; MS m/z 550 [M + Na]+; HRMS calcd for C29H58O4NaSi2 [M + Na]+ 549.3766, found 549.3758.2-((4S,5S)-2,2-Dimethyl-5-((5R,6R,10R,11S,E)-2,2,3,3,6,10,11,13,13,14,14-undecamethyl-4,12-dioxa-3,13-disilapentadec-8-en-5-yl)-1,3-dioxolan-4-yl)ethanol (16)
Me2S–BH3 complex (0.03 mL, 0.29 mmol) was added dropwise to a stirring solution of cyclohexene (0.06 mL, 0.57 mmol) in THF (0.2 mL) at 0 °C. The resulting solution was stirred for 15 min, and a solution of compound 3 (100 mg, 0.19 mmol) in THF (2 mL) was then added at the same temperature. The solution was allowed to warm to rt, and all starting material was consumed within 1 h (reaction monitored by TLC). The reaction mixture was then treated with NaOH (3 N, 2 mL) followed by H2O2 (30%, 2 mL) at 0 °C and stirred for 2 h. The reaction mixture was extracted with EtOAc (3 × 5 mL), and the combined extracts were washed with brine (10 mL) solution, dried over anhydrous Na2SO4 and concentrated. the resultant residue was purified by silica gel column chromatography (EtOAc–hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) to afford 16 (72.6 mg, 70%) as a colorless oil; Rf = 0.5 (hexane–EtOAc, 8
9) to afford 16 (72.6 mg, 70%) as a colorless oil; Rf = 0.5 (hexane–EtOAc, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); [α]24D = −21.15 (c 1.4, CHCl3); IR (KBr): 3448, 2939, 1463, 1375, 1220, 1079, 838 cm−1; 1H NMR (500 MHz, CDCl3): δ 5.41–5.31 (m, 2H), 4.29 (ddd, J = 11.4, 5.2, 2.1 1H), 4.08 (dd, J = 8.1, 5.3 Hz, 1H), 3.88–3.79 (m, 3H), 3.72–3.66 (m, 1H), 2.56 (br s, 1H), 2.32–2.24 (m, 1H), 2.16–2.09 (m, 1H), 1.97–1.89 (m, 1H), 1.84–1.73 (m, 2H), 1.71–1.58 (m, 1H), 1.46 (s, 3H), 1.33 (s, 3H), 1.03 (d, J = 6.1 Hz, 3H), 0.99–0.95 (m, 6H), 0.88 (s, 9H), 0.86 (s, 9H), 0.08 (s, 3H), 0.05 (s, 3H), 0.04–0.02 (m, 6H) ppm; 13C NMR (75 MHz, CDCl3): δ 134.5, 129.1, 108.2, 78.4, 77.8, 72.4, 72.0, 61.7, 44.4, 39.0, 36.0, 32.1, 28.4, 25.9 (2C), 20.5, 18.2, 18.1, 16.2, 14.5, −3.6, −4.3, −4.4, −4.8 ppm; MS m/z 567 [M + Na]+; HRMS calcd for C29H60O5NaSi2 [M + Na]+ 567.3874, found 567.3874.
2); [α]24D = −21.15 (c 1.4, CHCl3); IR (KBr): 3448, 2939, 1463, 1375, 1220, 1079, 838 cm−1; 1H NMR (500 MHz, CDCl3): δ 5.41–5.31 (m, 2H), 4.29 (ddd, J = 11.4, 5.2, 2.1 1H), 4.08 (dd, J = 8.1, 5.3 Hz, 1H), 3.88–3.79 (m, 3H), 3.72–3.66 (m, 1H), 2.56 (br s, 1H), 2.32–2.24 (m, 1H), 2.16–2.09 (m, 1H), 1.97–1.89 (m, 1H), 1.84–1.73 (m, 2H), 1.71–1.58 (m, 1H), 1.46 (s, 3H), 1.33 (s, 3H), 1.03 (d, J = 6.1 Hz, 3H), 0.99–0.95 (m, 6H), 0.88 (s, 9H), 0.86 (s, 9H), 0.08 (s, 3H), 0.05 (s, 3H), 0.04–0.02 (m, 6H) ppm; 13C NMR (75 MHz, CDCl3): δ 134.5, 129.1, 108.2, 78.4, 77.8, 72.4, 72.0, 61.7, 44.4, 39.0, 36.0, 32.1, 28.4, 25.9 (2C), 20.5, 18.2, 18.1, 16.2, 14.5, −3.6, −4.3, −4.4, −4.8 ppm; MS m/z 567 [M + Na]+; HRMS calcd for C29H60O5NaSi2 [M + Na]+ 567.3874, found 567.3874.
2-((4S,5S)-2,2-Dimethyl-5-((5R,6R,10R,11S,E)-2,2,3,3,6,10,11,13,13,14,14-undecamethyl-4,12-dioxa-3,13-disilapentadec-8-en-5-yl)-1,3-dioxolan-4-yl)acetic acid (17)
A mixture of 16 (100 mg, 0.18 mmol), [bis(acetoxy)-iodo]benzenze (BAIB; 236 mg, 0.73 mmol) and 2,2,6,6-tetramethyl-1-piperidinyloxyl (TEMPO; 14.33 mg, 0.09 mmol) were stirred in CH3CN (1.5 mL) and H2O (0.5 mL) at rt for 2 h. The solution was quenched with 2 mL hypo solution and extracted with AcOEt (3 × 5 mL). After concentration under reduced pressure, the obtained residue was purified by column chromatography on silica gel (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 hexane–AcOEt) to afford compound 17 as a colorless viscous oil (90.1 mg, 88%); Rf = 0.5 (EtOAc–hexane, 1
2 hexane–AcOEt) to afford compound 17 as a colorless viscous oil (90.1 mg, 88%); Rf = 0.5 (EtOAc–hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); [α]24D = −38.8 (c 0.2, CHCl3); IR (KBr): 3448, 2956, 2929, 1712, 1465, 1377, 1253, 1080, 836 cm−1; 1H NMR (500 MHz, CDCl3): δ 5.42–5.31 (m, 2H), 4.55 (ddd, J = 10.7, 4.9, 3.1 Hz, 1H), 4.14 (dd, J = 7.3, 5.2 Hz, 1H), 3.79 (dd, J = 7.5, 3.5 Hz, 1H), 3.72–3.66 (m, 1H), 2.64–2.50 (m, 2H), 2.29–2.24 (m, 1H), 2.17–2.10 (m, 1H), 1.94–1.89 (m, 1H), 1.80–1.70 (m, 1H), 1.47 (s, 3H), 1.34 (s, 3H), 1.04 (d, J = 6.1 3H), 0.97 (d, J = 5.5 Hz, 3H), 0.96 (d, J = 5.3 Hz, 3H), 0.88 (s, 9H), 0.87 (s, 9H), 0.08 (s, 3H), 0.07 (s, 3H), 0.04–0.02 (m, 6H) ppm; 13C NMR (75 MHz, CDCl3): δ 176.4, 134.7, 128.7, 108.6, 77.1, 74.7, 72.6, 71.9, 44.4, 38.9, 36.7, 36.2, 28.5, 25.9 (2C), 20.6, 18.2, 18.1, 16.3, 14.5, −3.7, −4.3, −4.4, −4.8 ppm; MS m/z 567 [M + Na]+; HRMS calcd for C29H58O6NaSi2 [M + Na]+ 581.3664, found 581.3669.
1); [α]24D = −38.8 (c 0.2, CHCl3); IR (KBr): 3448, 2956, 2929, 1712, 1465, 1377, 1253, 1080, 836 cm−1; 1H NMR (500 MHz, CDCl3): δ 5.42–5.31 (m, 2H), 4.55 (ddd, J = 10.7, 4.9, 3.1 Hz, 1H), 4.14 (dd, J = 7.3, 5.2 Hz, 1H), 3.79 (dd, J = 7.5, 3.5 Hz, 1H), 3.72–3.66 (m, 1H), 2.64–2.50 (m, 2H), 2.29–2.24 (m, 1H), 2.17–2.10 (m, 1H), 1.94–1.89 (m, 1H), 1.80–1.70 (m, 1H), 1.47 (s, 3H), 1.34 (s, 3H), 1.04 (d, J = 6.1 3H), 0.97 (d, J = 5.5 Hz, 3H), 0.96 (d, J = 5.3 Hz, 3H), 0.88 (s, 9H), 0.87 (s, 9H), 0.08 (s, 3H), 0.07 (s, 3H), 0.04–0.02 (m, 6H) ppm; 13C NMR (75 MHz, CDCl3): δ 176.4, 134.7, 128.7, 108.6, 77.1, 74.7, 72.6, 71.9, 44.4, 38.9, 36.7, 36.2, 28.5, 25.9 (2C), 20.6, 18.2, 18.1, 16.3, 14.5, −3.7, −4.3, −4.4, −4.8 ppm; MS m/z 567 [M + Na]+; HRMS calcd for C29H58O6NaSi2 [M + Na]+ 581.3664, found 581.3669.
Mupirocin H (2)
Concentrated HCl (0.2 mL) was added to a stirring solution of acid 17 (40 mg, 0.07 mmol) in THF (5 mL), and the resulting solution was stirred at 65 °C for 6 h. The reaction mixture was diluted with CH2Cl2 (10 mL) and sat. aq. NaCl solution (10 mL). The organic layer was separated, and the aqueous layer was extracted with 10% MeOH–CH2Cl2 (4 × 10 mL). The combined organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo. Purification by silica gel column chromatography (MeOH–CH2Cl2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19) afforded mupirocin H 2 (16.55 mg, 85%) as a colorless oil; Rf = 0.3 (MeOH–CH2Cl2 1
19) afforded mupirocin H 2 (16.55 mg, 85%) as a colorless oil; Rf = 0.3 (MeOH–CH2Cl2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9); [α]24D = +23.3 (c 0.4, CHCl3); IR (KBr): 3419, 2926, 2855, 1771, 1456, 1377, 1067, 771 cm−1; 1H NMR (500 MHz, CDCl3): δ 5.61 (ddd, J = 15.0, 8.2, 6.3 Hz, 1H), 5.39 (dd, J = 15.4, 8.7 Hz, 1H), 4.62–4.56 (m, 1H), 4.42 (dd, J = 5.6, 3.2 Hz, 1H), 3.60–3.56 (m, 1H), 3.54–3.48 (m, 1H), 2.93 (dd, J = 18.2, 7.5 Hz, 1H), 2.52 (dd, J = 18.2, 4.4 Hz, 1H), 2.30–2.20 (m, 2H), 2.10–2.02 (m, 1H), 1.94–1.84 (m, 1H), 1.18 (d, J = 6.1 Hz, 3H), 1.05 (d, J = 7.0 Hz, 3H), 0.98 (d, J = 6.9 Hz 3H); 13C NMR (75 MHz, CDCl3): δ 175.8, 134.7, 129.5, 87.6, 75.1, 71.5, 68.4, 45.2, 38.2, 35.2, 34.5, 20.5, 16.8, 15.9 ppm; MS m/z 295 [M + Na]+; HRMS calcd for C14H24O5Na [M + Na]+ 295.1495, found 295.1516.
9); [α]24D = +23.3 (c 0.4, CHCl3); IR (KBr): 3419, 2926, 2855, 1771, 1456, 1377, 1067, 771 cm−1; 1H NMR (500 MHz, CDCl3): δ 5.61 (ddd, J = 15.0, 8.2, 6.3 Hz, 1H), 5.39 (dd, J = 15.4, 8.7 Hz, 1H), 4.62–4.56 (m, 1H), 4.42 (dd, J = 5.6, 3.2 Hz, 1H), 3.60–3.56 (m, 1H), 3.54–3.48 (m, 1H), 2.93 (dd, J = 18.2, 7.5 Hz, 1H), 2.52 (dd, J = 18.2, 4.4 Hz, 1H), 2.30–2.20 (m, 2H), 2.10–2.02 (m, 1H), 1.94–1.84 (m, 1H), 1.18 (d, J = 6.1 Hz, 3H), 1.05 (d, J = 7.0 Hz, 3H), 0.98 (d, J = 6.9 Hz 3H); 13C NMR (75 MHz, CDCl3): δ 175.8, 134.7, 129.5, 87.6, 75.1, 71.5, 68.4, 45.2, 38.2, 35.2, 34.5, 20.5, 16.8, 15.9 ppm; MS m/z 295 [M + Na]+; HRMS calcd for C14H24O5Na [M + Na]+ 295.1495, found 295.1516.
Acknowledgements
YS thanks CSIR, New Delhi for financial assistance in the form of a fellowship. NHK thanks NIPER, Hyderabad for financial assistance in the form of a fellowship. PSH acknowledges funding from CSIR, New Delhi as part of the XII Five year plan program under title ORIGIN (CSC-108) and research grant (P-81-113) from the Human Resources Research Group-New Delhi through the Council of Scientific & Industrial Research (CSIR) Young Scientist Award Scheme. This paper is dedicated to Dr. J. S. Yadav on the occasion of his 64th birthday.Notes and references
- M. H. Wilcox, J. Hall, H. Pike, P. A. Templeton, W. N. Fawley, P. Parnell and P. Verity, J. Hosp. Infect., 2003, 54, 196–201 CrossRef CAS.
- S. M. Cooper, R. J. Cox, J. Crosby, M. P. Crump, J. Hothersall, W. Laosripaiboon, T. J. Simpson and C. M. Thomas, Chem. Commun., 2005, 1179–1181 RSC.
- J. Wu, S. M. Cooper, R. J. Cox, J. Crosby, M. P. Crump, J. Hothersall, T. J. Simpson, C. M. Thomas and C. L. Willis, Chem. Commun., 2007, 2040–2042 RSC.
- (a) Y. J. Class and P. DeShong, Chem. Rev., 1995, 95, 1843 CrossRef CAS and references cited therein; (b) C. McKay, T. J. Simpson, C. L. Willis, A. K. Forrest and P. J. O’Hanlon, Chem. Commun., 2000, 1109 RSC; (c) T. Honda and N. Kimura, Org. Lett., 2002, 4, 4567 CrossRef CAS PubMed; (d) X. Gao and D. G. Hall, J. Am. Chem. Soc., 2005, 127, 1628 CrossRef CAS PubMed; (e) L. van Innis, J. M. Plancher and I. E. Marko, Org. Lett., 2006, 8, 6111 CrossRef CAS PubMed; (f) R. F. de la Pradilla and N. Lwoff, Tetrahedron Lett., 2008, 49, 4167 CrossRef PubMed; (g) R. F. de la Pradilla, N. Lwoff and A. Viso, Eur. J. Org. Chem., 2009, 2312 CrossRef CAS PubMed; (h) S. P. Udawant and T. K. Chakraborty, J. Org. Chem., 2011, 76, 6331 CrossRef CAS PubMed; (i) R. W. Scott, C. Mazzetti, T. J. Simpson and C. L. Willis, Chem. Commun., 2012, 48, 2639 RSC.
- (a) S. Udawant and T. K. Chakraborty, J. Org. Chem., 2011, 76, 6331–6337 CrossRef CAS PubMed; (b) R. W. Scott, C. Mazzetti, T. J. Simpson and C. L. Willis, Chem. Commun., 2012, 48, 2639–2641 RSC; (c) C. Zhao, Z. Yuan, Y. Zhang, B. Ma, H. Li, S. Tang, X. Xie and X. She, Org. Chem. Front., 2014, 1, 105–108 RSC.
- (a) Y. Sridhar and P. Srihari, Org. Biomol. Chem., 2013, 11, 4640–4645 RSC; (b) Y. Sridhar and P. Srihari, Eur. J. Org. Chem., 2013, 578–587 CrossRef CAS PubMed; (c) P. Srihari, A. Sathish Reddy, J. S. Yadav, D. Yedlapudi and V. S. Kalivendi, Tetrahedron Lett., 2013, 54, 5616–5618 CrossRef CAS PubMed; (d) P. Srihari and Y. Sridhar, Eur. J. Org. Chem., 2011, 6690–6697 CrossRef CAS PubMed; (e) P. Srihari, K. Satyanarayana, B. Ganganna and J. S. Yadav, J. Org. Chem., 2011, 76, 1922–1925 CrossRef PubMed.
- Y. Sridhar and P. Srihari, Org. Biomol. Chem., 2014, 12, 2950–2959 CAS.
- P. Srihari, B. Kumaraswamy and J. S. Yadav, Tetrahedron, 2009, 65, 6304–6309 CrossRef CAS PubMed.
- The same product was synthesized in 5 steps starting from d-ribose. See L.-S. Li and D.-R. Hou, RSC Adv., 2014, 4, 91–97 RSC.
- (a) K. Omura and D. Swern, Tetrahedron, 1978, 34, 1651–1660 CrossRef CAS; (b) K. Vamshikrishna and P. Srihari, Tetrahedron, 2012, 68, 1540–1546 CrossRef CAS PubMed.
- T. Andreou, A. M. Costa, L. Esteban, L. Gonzalez, G. Mas and J. Vilarrasa, Org. Lett., 2005, 7, 4083–4086 CrossRef CAS PubMed.
- S. R. Harutyunyan, T. d. Hartog, K. Geurts, A. J. Minnaard and B. L. Feringa, Chem. Rev., 2008, 108, 2824–2852 CrossRef CAS PubMed and references cited there in.
- A. S. Kireev, M. Manpadi and A. Kornienko, J. Org. Chem., 2006, 71, 2630–2640 CrossRef CAS PubMed.
- (a) D. B. Dess and J. C. Martin, J. Am. Chem. Soc., 1991, 113, 7277–7287 CrossRef CAS; (b) J. S. Yadav and P. Srihari, Tetrahedron: Asymmetry, 2004, 15, 81–89 CrossRef CAS PubMed.
- (a) W. Oppolzer, J. Blagg, I. Rodriguez and E. Walther, J. Am. Chem. Soc., 1990, 112, 2767–2772 CrossRef CAS; (b) W. Oppolzer, C. Starkemann, I. Rodriguez and G. Bernardinelli, Tetrahedron Lett., 1991, 32, 61–64 CrossRef CAS . Procedure was followed from ref. 7.
- O. Mitsunobu, Synthesis, 1981, 1–28 CrossRef CAS.
- (a) J. B. Baudin, G. Hareau, S. A. Julia and O. Ruel, Tetrahedron Lett., 1991, 32, 1175–1178 CrossRef CAS; (b) C. Aïssa, Eur. J. Org. Chem., 2009, 1831–1844 CrossRef PubMed.
- S. K. Kang, K. Y. Jung, J. U. Chung, E. Y. Namkoong and T. H. Kim, J. Org. Chem., 1995, 60, 4678–4679 CrossRef CAS.
- Initial attempts with BH3·DMS and 9-BBN ended with recovery of the starting material.
- (a) H. C. Brown, A. K. Mandal and S. U. Kulkarni, J. Org. Chem., 1977, 42, 1392–1398 CrossRef CAS; (b) M. M. Midland and Y. C. Kwon, J. Am. Chem. Soc., 1983, 105, 3725–3727 CrossRef CAS.
- J. B. Epp and T. S. Widlanski, J. Org. Chem., 1999, 64, 293–295 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR and 13C NMR copies of new compounds. See DOI: 10.1039/c4ra06373b |
| This journal is © The Royal Society of Chemistry 2014 |