Entrapment in polymeric material of resting cells of Aspergillus flavus with lipase activity. Application to the synthesis of ethyl laurate
Abstract
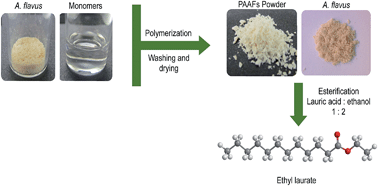
The lipase activity of resting cells of Aspergillus flavus was improved by entrapment in various polymeric acrylates (PAAFs). Commercially available ethyl acrylate, butyl acrylate and ethyl methacrylate, and acrylates synthesized from 1,3-dichloropropan-2-ol were used as monomers for the in situ polymerization. The cells were physically entrapped via free-radical-polymerization in aqueous medium or by bulk polymerization. The percentage of resting cells immobilized in the polymers was assessed by analysing the ergosterol content. Bulk polymerization with ethyl methacrylate allowed the greatest incorporation ratio, with 85% of entrapped biocatalyst. Entrapped resting cells were used to prepare ethyl laurate, achieving yields of up to 98%. The specific activity (r) of PAAFs was determined using a batch reactor and a packed-bed reactor (PBR). The highest r was observed for the resting cells entrapped in poly(ethyl methacrylate) using PBR. These results demonstrate that entrapped resting cells of A. flavus can be used to prepare commercial products.

 Please wait while we load your content...
Please wait while we load your content...