Current perspective on bacterial pigments: emerging sustainable compounds with coloring and biological properties for the industry – an incisive evaluation
Abstract
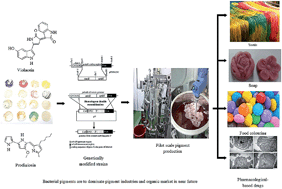
The current inclination towards exploiting bacterial pigments for various coloring functions, like food, cloth, painting, cosmetics, pharmaceuticals, plastics etc. is a well-recognized aspect. Nevertheless, the current bacterial pigment productions are not effective to meet their industrial needs. Current research going on world over on bacterial pigments signify that genetic engineering for strain improvement, optimization of bioprocess modelling and utilizing cheap agro-industrial residues as substrates are key developmental strategies to maximize pigment production from bacteria. Incidentally the superior performance characteristics of the bacteria for producing differing colouring compounds and the environmental acceptability of bacterial pigments are very encouraging factors to promote higher pigment production taking advantage of the current developmental strategies. This paper evaluates the current advances in bacterial pigment production, its recovery and wide-ranging scope of its industrial applications and commercial viability.

 Please wait while we load your content...
Please wait while we load your content...