Fabrication and evaluation of nanofibrous membranes with photo-induced chemical and biological decontamination functions
Abstract
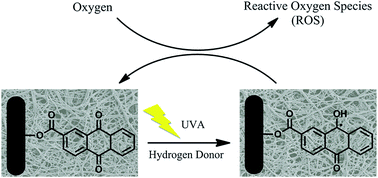
Hydrophilic nanofibrous membranes with photo-induced self-cleaning functionality were successfully prepared by covalently incorporating a photo-active anthraquinone derivative onto the surfaces of poly(vinyl alcohol-co-ethylene) (PVA-co-PE) nanofibrous membranes. The presence of ester bonds between anthraquinone structures and nanofiber surfaces, as evident from ATR-FTIR results, confirmed the desired surface functionalization. SEM images revealed that the prepared membranes possessed uniform nanofibers and ultrafine porous structures that remained intact after surface functionalization. Under UVA irradiation, these photo-active compound-modified nanofibrous membranes are able to generate reactive oxygen species (ROS), to which are attributed the observed self-decontamination functions. The generation of ROS was evaluated via quantitative determination of hydrogen peroxide on the membrane surfaces by using an iodometric titration method. The prepared hydrophilic nanofibrous membranes exhibited powerful photo-induced chemical and biological decontamination functions, including over 99.999% reduction against both Escherichia coli and Staphylococcus aureus, and a total decontamination of aldicarb, a carbamate pesticide, under light irradiation within a short contact time.

 Please wait while we load your content...
Please wait while we load your content...