Open Access Article
Open Access ArticlePoly(N-isopropylacrylamide) microgel-based thin film actuators for humidity sensing
Molla R. Islam and
Michael J. Serpe*
and
Michael J. Serpe*
Department of Chemistry, University of Alberta, Edmonton, AB T6G 2G2, Canada. E-mail: michael.serpe@ualberta.ca
First published on 23rd July 2014
Abstract
In this submission we fabricated a humidity-responsive polymer-based actuator by layering negatively charged poly(N-isopropylacrylamide)-co-acrylic acid microgels and positively charged poly(diallyldimethyl ammonium chloride) on top of a flexible plastic substrate. We show that the extent of the actuation (bending) was dependent on the atmospheric humidity. This property was used to detect atmospheric humidity by hanging weights from the actuator, which were rested on the pan of a top loading balance. This was done in such a way that the amount of the mass resting on the balance depended on the extent of actuator bending, which could then be related to humidity. We found the mass read by the balance to be linearly dependent on the atmospheric humidity in the range of 0–50% humidity, and the devices could be used to detect 0.02% humidity changes. These experiments show that the actuation of the devices can be used for sensing applications, in this case humidity, but will be used in the future to detect various atmospheric gases and other vapors.
Introduction
Stimuli responsive (or smart) polymers are capable of translating molecular changes into observable responses. These materials have found numerous applications, including sensing and biosensing.1 The most common response of responsive polymers is a conformational change; the polymer (or material made of the polymer) can undergo a change from one conformational state to another. This change is ideally reversible, i.e., when the stimulus is withdrawn, the polymer (and material) reverts back to its initial state.One of the most influential synthetic stimuli responsive polymers of our time is poly(N-isopropylacrylamide) (pNIPAm), which is a thermoresponsive polymer that undergoes a conformational change in water from an extended coil to compact globule when the temperature is raised >32 °C.2 More specifically, in water at T < 32 °C, pNIPAm exists in a “hydrophilic”, extended state, while at T > 32 °C, the pNIPAm's polymer–polymer interactions dominate, and the polymer chain becomes dehydrated and collapses. Additionally, crosslinked networks of pNIPAm can be synthesized, which are known as hydrogels. These networks are likewise thermoresponsive, undergoing a fully solvated to desolvated transition as the temperature of the water they are in is increased to >32 °C. Finally, colloidally stable hydrogel micro and nanoparticles can be synthesized (microgels and nanogels, respectively) and also exhibit the same temperature responsivity.
Interestingly, pNIPAm-based materials can be made responsive to other stimuli, in addition to temperature. This can be easily accomplished via copolymerization of other functional monomers into the systems at the time of synthesis. The most common functionalities added are amines and carboxylic acids; for example, acrylic acid (AAc), methacrylic acid (MAAc), vinyl acetic acid and N-(3-aminopropyl) methacrylamide hydrochloride (APMAH).3 Our group has used pNIPAm-co-AAc microgels for a number of different sensing applications.4 This is partially due to the ability of the AAc moieties to become negatively charged at pH above 4.25 (pKa of AAc ≈4.25) due to their deprotonation. The deprotonated AAc groups cause the microgels to swell due to Coulombic repulsion between the AAc moieties, and associated osmotic swelling. Interestingly, addition of positively charged polymers (polycation) to the negatively charged microgels results in their deswelling as a result of electrostatically induced intramicrogel crosslinking.5
Humidity is related to the amount of water in air, which is dependent on many factors, and influences many properties, including human comfort and health. For industrial processes, humidity is a property that must be closely monitored and controlled, and can have broad implications.6 For example, the quality of thin film deposition for the electronic industry, e.g., for memory devices, is greatly influenced by humidity. Therefore, new devices for measuring humidity in a variety of environments are needed.7 In this submission, pNIPAm microgel-based devices were fabricated that bend to different extents depending on the humidity of the environment they are exposed to. The devices were fabricated by layering a metal layer and two polymer layers on top of a plastic substrate. Due to the electrostatic interactions between the polymer layers, and their adhesion to the plastic substrate, their solvation state changes at different humidity results in device bending. We have previously shown that these devices are capable of lifting masses many times their own mass, which we exploit here for sensing environmental humidity.8
Experimental
Materials
N-Isopropylacrylamide was purchased from TCI (Portland, Oregon) and purified by recrystallization from hexanes (ACS reagent grade, EMD, Gibbstown, NJ) prior to use. N,N′-methylenebisacrylamide (BIS) (99%), acrylic acid (AAc) (99%), and ammonium persulfate (APS) (98+%) were obtained from Sigma-Aldrich (Oakville, Ontario) and were used as received. Poly(diallyldimethylammonium chloride) solution (pDADMAC) of MW < 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (20% in water) was purchased from Sigma-Aldrich (St. Louis, MO). Deionized (DI) water with a resistivity of 18.2 MΩ cm was used. Cr/Au annealing was done in a Thermolyne muffle furnace from Thermo Fisher Scientific (Ottawa, Ontario). Anhydrous ethanol was obtained from Commercial Alcohols (Brampton, Ontario). Fisher's finest glass coverslips were 25 × 25 mm and obtained from Fisher Scientific (Ottawa, Ontario). Cr was 99.999% and obtained from ESPI (Ashland, OR), while Au was 99.99% and obtained from MRCS Canada (Edmonton, AB).
000 (20% in water) was purchased from Sigma-Aldrich (St. Louis, MO). Deionized (DI) water with a resistivity of 18.2 MΩ cm was used. Cr/Au annealing was done in a Thermolyne muffle furnace from Thermo Fisher Scientific (Ottawa, Ontario). Anhydrous ethanol was obtained from Commercial Alcohols (Brampton, Ontario). Fisher's finest glass coverslips were 25 × 25 mm and obtained from Fisher Scientific (Ottawa, Ontario). Cr was 99.999% and obtained from ESPI (Ashland, OR), while Au was 99.99% and obtained from MRCS Canada (Edmonton, AB).
Poly(N-isopropylacrylamide)-co-acrylic acid microgel synthesis
Microgels composed of poly(N-isopropylacrylamide)-co-acrylic acid (pNIPAm-co-AAc) were synthesized via temperature-ramp, surfactant free, free radical precipitation polymerization as described previously.3f,9 The pregel solution of monomer mixture, was comprised of 85% (mole mole−1) NIPAm, 10% AAc, and 5% BIS as the crosslinker. NIPAm (17.0 mmol), and BIS (1.0 mmol) were dissolved in deionized water (100 mL) with stirring in a beaker. The mixture was filtered through a 0.2 μm filter affixed to a 20 mL syringe into a 200 mL 3-neck round-bottom flask. The beaker was rinsed with 25 mL of deionized water and then filtered into the NIPAm/BIS solution. The flask was then equipped with a temperature probe, a condenser and a N2 gas inlet. The solution was bubbled with N2 gas for ∼1.5 h, while stirring at a rate of 450 RPM, allowing the temperature to reach 45 °C. AAc (2.0 mmol) was then added to the heated mixture with a micropipette in one aliquot. A 0.078 M aqueous solution of APS (5 mL) was delivered to the reaction flask with a transfer pipet to initiate the reaction. Immediately following initiation, a temperature ramp of 45 to 65 °C was applied to the solution at a rate of 30 °C h−1. The reaction was allowed to proceed overnight at 65 °C. After polymerization, the reaction mixture was allowed to cool down to room temperature and filtered through glass wool to remove any large aggregates. The coagulum was rinsed with deionized water and filtered. Aliquots of these microgels (14 mL) were centrifuged at a speed of ∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 relative centrifugal force (RCF) at 23 °C for ∼40 minutes to produce a pellet at the bottom of the centrifuge tube. The supernatant was removed from the pellet of microgels, which was then re-suspended to the original volume (14 mL) using deionized water. This process was repeated until the microgels were cleaned a total of six times to remove any unreacted monomer and/or linear polymer from the microgel solution. The diameter of the microgel particles was measured to be ≈1.5 μm by a differential interference contrast microscope (data not shown).
000 relative centrifugal force (RCF) at 23 °C for ∼40 minutes to produce a pellet at the bottom of the centrifuge tube. The supernatant was removed from the pellet of microgels, which was then re-suspended to the original volume (14 mL) using deionized water. This process was repeated until the microgels were cleaned a total of six times to remove any unreacted monomer and/or linear polymer from the microgel solution. The diameter of the microgel particles was measured to be ≈1.5 μm by a differential interference contrast microscope (data not shown).
Device fabrication
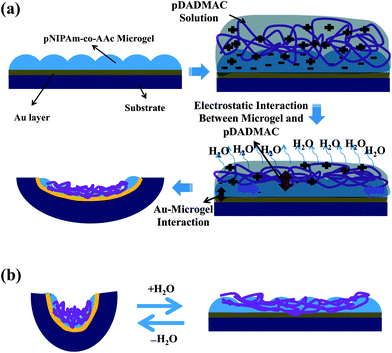
Transparent flexible plastic substrate (transparency films for laser copiers from 3 M company, Canada) were rinsed with DI water and ethanol and dried with N2 gas, and 2 nm of Cr followed by 15 nm or 50 nm of Au were thermally evaporated onto them at a rate of ∼0.2 Å s−1 and ∼0.1 Å s−1, respectively, using a Torr International Inc. model THEUPG thermal evaporation system (New Windsor, NY). The Cr acts as adhesion layer to hold the Au layer on the plastic. An aliquot of about 14 mL of previously purified microgel solution was centrifuged for 30 min at 23 °C at ∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 RCF to pack the microgels into a pellet at the bottom of the tube. After removal of the supernatant solution, the microgel pellet was vortexed and placed onto a hot plate at 30 °C. An aliquot (40 μL for each 2.54 cm2) of the concentrated microgels was put onto the substrate and then spread toward each edge using the side of a micropipette tip. The substrate was rotated, and the microgel solution was spread again to avoid uneven drying. The spreading and rotation continued until the microgel solution became too viscous to spread due to drying. The microgel solution was allowed to dry completely on the substrate for 2 h with the hot plate temperature set to 30 °C. After 2 h, the dry film was rinsed copiously with DI water to remove any excess microgels not bound directly to the Au. The microgel coated substrate was then placed into a DI water bath and allowed to incubate overnight on a hot plate set to ∼30 °C. Following this step, the substrate was again rinsed with DI water to further remove any microgels not bound directly to the Au substrate surface. The microgel painted Au coated substrate was dried with N2 gas and a specific amount of a pDADMAC solution (20% in water) was spread onto the microgel layer and was undisturbed and dried at ambient temperature. PDADMAC is a polycation, therefore, the pDADMAC layer crosslinks (and binds) with the microgels bound to the Au layer. As the drying continues, the pDADMAC layer contracts, and since it is attached to the microgels electrostatically, which is tightly adhered to the plastic via the Au, the device curls up. This behavior has been previously described by our group.8a
000 RCF to pack the microgels into a pellet at the bottom of the tube. After removal of the supernatant solution, the microgel pellet was vortexed and placed onto a hot plate at 30 °C. An aliquot (40 μL for each 2.54 cm2) of the concentrated microgels was put onto the substrate and then spread toward each edge using the side of a micropipette tip. The substrate was rotated, and the microgel solution was spread again to avoid uneven drying. The spreading and rotation continued until the microgel solution became too viscous to spread due to drying. The microgel solution was allowed to dry completely on the substrate for 2 h with the hot plate temperature set to 30 °C. After 2 h, the dry film was rinsed copiously with DI water to remove any excess microgels not bound directly to the Au. The microgel coated substrate was then placed into a DI water bath and allowed to incubate overnight on a hot plate set to ∼30 °C. Following this step, the substrate was again rinsed with DI water to further remove any microgels not bound directly to the Au substrate surface. The microgel painted Au coated substrate was dried with N2 gas and a specific amount of a pDADMAC solution (20% in water) was spread onto the microgel layer and was undisturbed and dried at ambient temperature. PDADMAC is a polycation, therefore, the pDADMAC layer crosslinks (and binds) with the microgels bound to the Au layer. As the drying continues, the pDADMAC layer contracts, and since it is attached to the microgels electrostatically, which is tightly adhered to the plastic via the Au, the device curls up. This behavior has been previously described by our group.8a
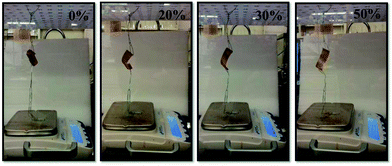
Paper clips were attached and hung from the end of the devices, and the device could lift up the mass; the extent of the lifting depends on humidity. For this study, the devices and a top loading balance by Denver Instrument (Model SI-114) were added to a chamber fitted with a humidity feedback system by ramé-hart Instrument Co. (Model 100-26 TH) that was capable of controlling humidity using an Air-O-Swiss AOS 7145 Cool Mist Ultrasonic humidifier (manufactured by Swiss Pure Air) and a humidity sensor from TEGAM (Model RDP-20C). Humid air, or dry air, was pumped into the chamber to maintain the set humidity. The chamber was fitted with a small fan to ensure the humidity in the chamber was evenly distributed. The paperclips were rested on the pan of the balance, and the mass of the paperclips on the balance measured as a function of humidity. That is, the mass of the paperclips on the balance pan could be varied with humidity.
Results and discussion
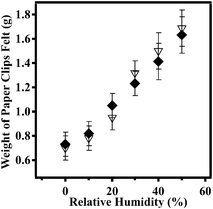
In previous study, we showed that a polymer-based artificial muscle/actuator can be constructed by first painting a monolithic microgel layer on top of a Au layer, supported on a plastic/bendable substrate.8a Deposition of a polycation (pDADMAC) on top of the microgel layer yields strong electrostatic interactions between the pDADMAC layer and the microgel layer adhered to the Au/plastic substrate. Therefore, when the pDADMAC layer contracts upon drying, the substrate bends. A cartoon depiction of the device structure and its bending can be seen in Fig. 1. It is important to point out that the extent of device bending is related to the atmospheric humidity, which is capable of altering the hydration of the pDADMAC. That is, high humidity swells the pDADMAC layer, which causes the device to open, while dry air dehydrates the pDADMAC layer causing the device to close/bend. This behavior has allowed masses to be lifted as a function of humidity, which are many times the mass of the device itself.In this study, we show that the ability of the devices to lift masses can be sued for sensing applications, as we show here using the devices to sense humidity. The device was constructed following the protocol published previously, and detailed above.8a Once constructed, the device was added to a humidity-controlled chamber and hung from a support. Paperclips (used as weights) were then hung from the device, and rested on the pan of a top loading balance also in the humidity-controlled chamber. A photograph of the setup can be seen in Fig. 2. Initially, the chamber was held at 0% relative humidity, which causes the device to be completely curled up, as can be seen in Fig. 2. In this case, the weights are pulled off of the balance's pan, which records a low mass. We found that at 0% humidity the weight felt by the balance was 0.63 g and stayed stable at fixed humidity. When the relative humidity was increased from 0% to 10% (usually takes 30 minutes to fully stabilize) the device opened up, which allowed more of the paperclips to be added to the balance pan and subsequently increasing the mass felt by the balance to 0.72 g. As the chamber humidity was incrementally increased, we found that the device opens up, subsequently adding more of the paperclips to the balance pan. At 50% relative humidity, the device is completely opened, bringing the mass of the paperclips up to 1.34 g. By fitting a line to the data, a slope of 0.02 ± 0.01 g% humidity−1 is obtained. Since the top loading balance is capable of measuring 0.01 g, 0.02% humidity changes can be measured. The complete response of the device over the whole humidity range is shown in Fig. 3. Therefore, we were able to correlate the change in weight felt by the balance with the relative humidity of the chamber. Additionally, we showed that the response of the device to chamber humidity was reversible, i.e., once the chamber humidity was decreased the device was able to curl back up, removing the paperclip mass from the balance pan. The reversibility can be seen in Fig. 3.
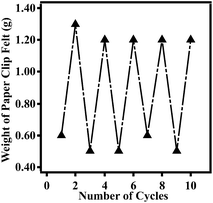
Finally, we were able to show that the same device was able to actuate multiple times, therefore making the device capable of sensing relative humidity in a real time and repeatable fashion. To do this, the chamber humidity was cycled between 0% and 50% relative humidity, while monitoring the mass of the paperclips on the pan. As can be seen in Fig. 4, the device is capable of responding to many cycles of varying humidities.
In summary, we were able to show that the humidity-mediated response of polymer-based actuators could be used for sensing atmospheric humidity. This was done by attaching masses to the end of the device, which were rested on the pan of a top loading balance. The various extents of device curling, mediated by humidity, could be correlated to the mass felt by the balance, which was linearly related to atmospheric humidity over the range of 0–50% relative humidity. We also showed that the device's response was dynamic, and capable of real time monitoring of atmospheric humidity over many high and low humidity cycles. We strongly believe that the use of molecular electrostatic interaction will help build a low cost humidity sensor without any complicated setup, which can be used in the future for sensing other atmospheric components.
Acknowledgements
M.J.S. acknowledges funding from the University of Alberta (the Department of Chemistry and the Faculty of Science), the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canada Foundation for Innovation (CFI), the Alberta Advanced Education & Technology Small Equipment Grants Program (AET/SEGP) and Grand Challenges Canada. M.J.S. acknowledges Mark McDermott for the use of the thermal evaporator.References
- (a) E. Kokufata, Y. Q. Zhang and T. Tanaka, Nature, 1991, 351, 302 CrossRef CAS; (b) K. Tsujii, M. Hayakawa, T. Onda and T. Tanaka, Trans. Mater. Res. Soc. Jpn., 2001, 26, 639 CAS; (c) A. B. W. Brochu, S. L. Craig and W. M. Reichert, J. Biomed. Mater. Res., Part A, 2011, 96A, 492 CrossRef CAS PubMed; (d) J. M. Lenhardt and S. L. Craig, Nat. Nanotechnol., 2009, 4, 284 CrossRef CAS PubMed; (e) D. M. Loveless, N. I. Abu-Lail, M. Kaholek, S. Zauscher and S. L. Craig, Angew. Chem., Int. Ed., 2006, 45, 7812 CrossRef CAS PubMed; (f) L. Brunsveld, E. W. Meijer, R. B. Prince and J. S. Moore, J. Am. Chem. Soc., 2001, 123, 7978 CrossRef CAS PubMed; (g) S. R. White, N. R. Sottos, P. H. Geubelle, J. S. Moore, M. R. Kessler, S. R. Sriram, E. N. Brown and S. Viswanathan, Nature, 2002, 415, 817 CrossRef CAS; (h) R. Pelton, Adv. Colloid Interface Sci., 2000, 85, 1 CrossRef CAS.
- (a) E. C. Cho, J. Lee and K. Cho, Macromolecules, 2003, 36, 9929 CrossRef CAS; (b) C. Wu, Polymer, 1998, 39, 4609 CrossRef CAS; (c) C. Wu and S. Q. Zhou, Macromolecules, 1996, 29, 1574 CrossRef CAS.
- (a) W. H. Blackburn, E. B. Dickerson, M. H. Smith, J. F. McDonald and L. A. Lyon, Bioconjugate Chem., 2009, 20, 960 CrossRef CAS PubMed; (b) J. Kim, S. Nayak and L. A. Lyon, J. Am. Chem. Soc., 2005, 127, 9588 CrossRef CAS PubMed; (c) S. Nayak, H. Lee, J. Chmielewski and L. A. Lyon, J. Am. Chem. Soc., 2004, 126, 10258 CrossRef CAS PubMed; (d) T. Hoare and R. Pelton, Macromolecules, 2004, 37, 2544 CrossRef CAS; (e) K. Kratz, T. Hellweg and W. Eimer, Colloids Surf., A, 2000, 170, 137 CrossRef CAS; (f) C. D. Sorrell and M. J. Serpe, Adv. Mater., 2011, 23, 4088 CrossRef CAS PubMed.
- (a) M. R. Islam, K. C. Johnson and M. J. Serpe, Anal. Chim. Acta, 2013, 792, 110 CrossRef CAS PubMed; (b) M. R. Islam and M. J. Serpe, Biosens. Bioelectron., 2013, 49, 133 CrossRef CAS PubMed; (c) M. R. Islam and M. J. Serpe, Chem. Commun., 2013, 49, 2646 RSC.
- (a) M. R. Islam and M. J. Serpe, Macromolecules, 2013, 46, 1599–1606 CrossRef CAS; (b) J. Kleinen, A. Klee and W. Richtering, Langmuir, 2010, 26, 11258 CrossRef CAS PubMed.
- N. Herzer, H. Guneysu, D. J. D. Davies, D. Yildirim, A. R. Vaccaro, D. J. Broer, C. W. M. Bastiaansen and A. P. H. J. Schenning, J. Am. Chem. Soc., 2012, 134, 7608 CrossRef CAS PubMed.
- E. Kim, S. Y. Kim, G. Jo, S. Kim and M. J. Park, ACS Appl. Mater. Interfaces, 2012, 4, 5179 CAS.
- (a) M. R. Islam, X. Li, K. Smyth and M. J. Serpe, Angew. Chem., Int. Ed., 2013, 52, 10330 CrossRef CAS PubMed; (b) X. Li and M. J. Serpe, Adv. Funct. Mater., 2014, 24, 4119 CrossRef CAS.
- Z. Meng, M. H. Smith and L. A. Lyon, Colloid Polym. Sci., 2009, 287, 277 CAS.
| This journal is © The Royal Society of Chemistry 2014 |