Electrospun nanofibers of p-type BiFeO3/n-type TiO2 hetero-junctions with enhanced visible-light photocatalytic activity
Y. C. Yang,
Y. Liu,
J. H. Wei*,
C. X. Pan,
R. Xiong and
J. Shi
Key Laboratory of Artificial Micro- and Nano-structures of Ministry of Education and School of Physics and Technology, Wuhan University, Wuhan 430072, China. E-mail: jhwei@whu.edu.cn
First published on 4th July 2014
Abstract
One-dimensional BiFeO3/TiO2 heterostructure nanofibers with high visible-light photocatalytic activity have been successfully obtained via a facile hydrothermal process followed by an electrospinning technique. The results show that the BiFeO3/TiO2 nanofibers are as long as dozens of micrometers with the diameters of about 100–300 nm, where BiFeO3 nanoparticles are surrounded by anatase-type TiO2 nanocrystals. Compared with the corresponding pure BiFeO3 nanoparticles, and TiO2 nanofibers, the as-prepared BiFeO3/TiO2 nanofibers exhibit a markedly enhanced photocatalytic activity in the degradation of methyl blue under visible light irradiation. The enhanced photocatalytic activity is attributed to the formed p–n heterojunction between BiFeO3 and TiO2, which results in synergistic enhancement. Notably, the BiFeO3/TiO2 nanofibers could be easily recycled without the decrease in the photocatalytic activity because of their one-dimensional nanostructural property. With their high degradation efficiency and fine recyclability, the BiFeO3/TiO2 heterostructure nanofibers will have wide application in photodegradation of various organic pollutants.
Introduction
During the previous decades, photocatalysis, as a “green” technique, has been extensively used in the area of environmental remediation because various kinds of pollutants can be decomposed completely by photocatalytically active semiconductors under light irradiation.1–3 Among those nanostructural semiconductor metal oxides, Titania (TiO2) nanomaterials, naturally n-type semiconductors with a wide bandgap (Eg = 3.2 eV for anatase), have been extensively studied owing to their excellent photo-chemical stability, low cost and non-toxicity.4,5 Nevertheless, the currently available TiO2 photocatalysts generally suffer from large band gap energy and rapid recombination of photo-induced carrier, which significantly deteriorate the photocatalytic activity. As a result, many methods have been made to extend the photo-response of the TiO2 to visible region and suppress the recombination of the photo-induced carriers. Research found that TiO2 doped with nonmetal (such as N, S, C, F, B, etc.6–9) and metal (such as Ag, Au, Cu, Pt, etc.10–14) could obviously extend the photo-response of the TiO2 to visible region and corresponding results in higher photocatalytic activity when it is illuminated under visible light. However, although the above modifications could partly improve the photocatalytic activity of TiO2, some key problems remain unresolved. For example, doped materials suffer from thermal instability, photocorrosion, lattice distortion, and an increase in the carrier recombination probability.15,16Another strategy to increase the visible light activity of titanium is to couple TiO2 with other narrow bandgap semiconductors. Compared to a single semiconductor, coupled semiconductors forms a hetero-junction structure which can transfer electrons from an excited small band gap semiconductor into another attached one in the case of proper band potentials. Such as In2O3,17 Bi2MoO6,18 Bi2WO6,19 CoFe2O4 sensitized20 or Ag–CdS21 co-sensitized TiO2 heterostructures have been reported to favor for the separation of photoinduced electrons and holes and thus dramatically improve the photocatalytic efficiency of the semiconductor heterostructures.
Multiferric BiFeO3 has been widely investigated due to its potential applications in information storage, spintronics, sensors and photocatalyst because of its narrow bandgap (∼2.0–2.8 eV).21–24 Recently, Li et al. reported that BiFeO3/TiO2 core–shell nanocrystals exhibited a high activity for photodegradation of Congo red because of the enhancement of the quantum efficiency by effectively promotion of electron and hole separation.25 However, the disadvantage of the structure is that the suspended particulates are easily lost in the process of photocatalytic reaction and separation, which may re-pollute the treated environment. Rohrer et al.26 have fabricated heterostructures of thin TiO2 film on BiFeO3 substrates by pulsed laser deposition, which can efficiently photo-reduce aqueous silver cations into silver nanoparticles under visible-light irradiation, but its photocatalytic activity is limited because of the low surface area.
As a contrast to random nanoparticles or thin films, the one-dimensional nanostructure such as nanowire, nanorod or nanofiber always show superior optoelectrical and photocatalytic property, and better dispersion owing to their excellent mobility of charge carriers or higher surface areas. For instance, the TiO2 nanofibers or modified TiO2 nanotubes have been of immense interest in recent years due to their enhanced photocatalytic activity and favorable recycling characteristics.27–30
Electrospinning, as an economical and simple method that is capable of fabricating nanofibers with high specific surface area and porous structure on a large scale, has been employed in many applications.31,32 To the best of our knowledge, the photocatalysis performances based on the p-type BiFeO3/n-type TiO2 nanofibers heterojunctions have not been explored until now. Herein, motivated by the above concerns, we fabricate one-dimensional BiFeO3/TiO2 nanofiber photocatalysts by combining the electrospinning technique with the hydrothermal method. The enhanced photocatalytic performance of BiFeO3/TiO2 nanofibers is attributed to fine light absorption capability of BiFeO3 and excellent charge separation characteristics of the formed heterojunction between BiFeO3 and TiO2. Besides, the BiFeO3/TiO2 nanofibers could be easily recycled without the decrease in the photocatalytic activity because of the large length to diameter ratio of nanofibers.
Experimental
Synthesis of BiFeO3/TiO2 heterostructure nanofibers
The composites were fabricated via a three-step process: (1) the BiFeO3 nanoparticles were prepared via the combining of coprecipitation method and hydrothermal method. A typical process as following: at first, 0.01 mol Fe (NO3)3·9H2O and 0.01 mol Bi (NO3)3·5H2O were dissolved in 50 mL of distilled water, then, 2 M KOH was added dropwise into the mixture to adjust the pH value of the solution and also serve as a mineralizer. The mixture was then aged at room temperature for 2 h. After that, 2 mL H2O2 was added into the solution and it was sealed in the autoclave as quickly as possible. Finally, it was heated at 200 °C for 24 h before it was furnace-cooled to room temperature. A gray powder obtained after filtration was washed with distilled water and dried in air. (2) BiFeO3/TiO2 heterostructure nanofibers were synthesized by an electrospinning technology. Typically, a quantity of tetra-butyl titanate (TBOT) and 1 g BiFeO3 seeds were added into a mixture of 40 mL ethanol and 10 mL acetic acid by magnetic stirring to form a biphase precursor. Then, 4 g of polyvinyl-pyrrolidone (PVP) was dissolved into the mixture by slowly stirring 6 h at room temperature. As followed, the mixture precursor solution was loaded into the 5 mL plastic syringe equipped with a 0.4 mm inner-diameter stainless steel, which was connected with an accelerating voltage of around 12 kV. The ultrafine nanofibers were collected in tinfoil substrate, with feeding rate of 30 μL min−1 and needle tip-to-collector distance of 10 cm. (3) Finally, the obtained composite nanofibers were annealed at 450 °C for 2 h in air atmosphere with the heating rate of 10°C min−1. The BiFeO3-doping concentration (X) was chosen as 5, 8, 10, 15, 20, which was the mole percentage of BiFeO3 in the theoretical TiO2 powders. The corresponding concentrations in the as-obtained photocatalysts were denoted as BTX. Unless otherwise specified, BiFeO3–TiO2 in the work refers to BT10. Pure TiO2 nanofibers were similarly prepared but without the addition of BiFeO3 seeds.Characterization
The powder X-ray diffraction (XRD) patterns of the samples were recorded on a D8 advance X-ray powder diffractometer (Germany) using CuKα radiation (λ = 0.1542 nm) in the 2θ range from 20° to 80°. The surface composition of the nanoparticles was evaluated by X-ray photoelectron spectra (XPS, ESCA-3400). Scanning electron microscope (SEM) images and selected-area electron diffraction (SAED) analyses were performed with a Sirion 200 field emission scanning electron microscope system at the accelerating voltage of 200 kV. Transmission electron microscope (TEM) and high-resolution transmission electron microscopy (HRTEM) images were performed on a JEOL JEM-2010 transmission electron microscopy (Japan) at the accelerating voltage of 200 kV equipped with energy dispersive X-ray spectrometer (EDX). UV-vis diffuse reflectance spectra (DRS) of the samples were obtained using an UV-vis spectrophotometer (Cary 5000 UV-vis-NIR, Australia) with an Integrated Sphere Attachment. All the measurements were performed at room temperature. The photocatalytic activities were evaluated by the degradation of MB in aqueous solution under visible-light irradiation (Xe lamp, 500 W; visible cutoff filter >420 nm). The reaction temperature was kept at room temperature to prevent any thermal catalytic effect. The photocurrents were measured by an electrochemical analyzer (CHI660A, CH Instruments Co.) in a standard three-electrode system using as-prepared samples as the working electrode, a saturated calomel electrode as reference, and a platinum wire parallel to the working electrode as a counter electrode. Photocurrent as a function of time was measured in aqueous NaSO4 (0.5 M) solution under visible light irradiation (λ> 420 nm). Electrochemical impedance spectroscopy (EIS) measurements were carried out in above configuration of cell under irradiation (λ> 420 nm) in a frequency range between 1 MHz and 0.1 Hz at applied biases is equal to −0.5 V. All measurements were conducted at room temperature and N2 atmosphere to obtain highly reproducible data.Photodegradation measurement
A self-made cylindrical glass vessel with a water-cooling jacket was used as a photochemical reactor. The photocatalytic activities of the as-prepared samples were evaluated by the degradation of a model pollutant dye solution, methylene blue (MB). The optical system for the photocatalytic reactions was composed of a 500 W xenon arc lamp and a cutoff filter (λ > 420 nm) at room temperature. Where, a 100 mL of the MB solution with an initial concentration of 10 mg L−1 in the presence of solid catalyst (50 mg) was stirred in the dark for 30 min to obtain a good dispersion and adsorption equilibrium. And then the mixed solution was illuminated under visible light in the photochemical reactor. At 30 min intervals of illumination, MB after photodegradation was analyzed at 660 nm as a function of time on a UV-vis spectrophotometer (Model: Shimadzu 2450/2550 PC). The BT10 samples were repeatedly used for five times with same experiment conditions to test the photocatalytic stability.Results and discussion
The structures of the as-obtained products were examined by X-ray powder diffraction (XRD), as shown in Fig. 1. It is revealed that the BiFeO3 nanoparticles are highly crystallized and exhibit a single-phase rhombohedral structure (Fig. 1a), all the peaks can be well indexed to a pure hexagonal structure with R3c space group for bismuth ferrite (JCPDS no. 86-1518). No other phases such as Bi2Fe4O9 and Bi2O3/Fe2O3 are detected in XRD spectra. Fig. 1b represents the X-ray diffraction pattern of as-obtained TiO2 sample. All diffraction peaks of TiO2 can be perfectly indexed by an anatase phase (JCPDS card no. 21-1272). No characteristic peaks of other impurities such as rutile and brookite were observed. The crystallite size of BiFeO3 and TiO2 were calculated to be 25.7 nm and 21.2 nm according to the broadening of corresponding X-ray spectral peaks by Scherrer formula: d = kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ. Here, d is the crystallite size, λ is the wavelength of the X-ray radiation (CuKα = 0.1542 nm), k is Scherrer constant, which is usually taken as 0.89, β is the line width at half-maximum height, and θ is the incidence angle. Fig. 1c represents the X-ray diffraction pattern of as-obtained BiFeO3/TiO2 sample (BT10). Both of the characteristic peaks of BiFeO3 (JCPDS no. 86-1518, hexagonal phase) and TiO2 (JCPDS card no. 21-1272, anatase) can be found in the pattern. This result confirmed the coexistence of BiFeO3 and TiO2 in the BiFeO3/TiO2 nanocomposites.
θ. Here, d is the crystallite size, λ is the wavelength of the X-ray radiation (CuKα = 0.1542 nm), k is Scherrer constant, which is usually taken as 0.89, β is the line width at half-maximum height, and θ is the incidence angle. Fig. 1c represents the X-ray diffraction pattern of as-obtained BiFeO3/TiO2 sample (BT10). Both of the characteristic peaks of BiFeO3 (JCPDS no. 86-1518, hexagonal phase) and TiO2 (JCPDS card no. 21-1272, anatase) can be found in the pattern. This result confirmed the coexistence of BiFeO3 and TiO2 in the BiFeO3/TiO2 nanocomposites.
XPS studies were further conducted to investigate the surface composition and chemical state of the prepared heteroarchitectures. Fig. 2 shows the fully scanned spectra of TiO2 and BT10 samples. The C element could be ascribed to the adventitious carbon-based contaminant, and the binding energy for C 1s peak at 284.6 eV was used as the reference for calibration. The survey XPS spectrum demonstrates the presence of Bi, O, Ti and Fe elements in the BT10 sample, suggesting a binary coexistence of TiO2 and BiFeO3 in the BT nanofibers. This result agrees well with XRD analysis. The typical high-resolution XPS spectra of O1s can be found in Fig. 2b. The peak at about 529.5 eV is due to oxygen in the TiO2 crystal lattice and the peak at about 531.0 eV is due to hydroxyl oxygen. The hydroxyl oxygen peak of the BT10 is more intensive than that of the undoped TiO2 and BiFeO3 samples, which means that BT10 sample maybe has better photocatalytic performance.
 | ||
| Fig. 2 (a) Survey XPS spectra of the TiO2 and BiFeO3/TiO2 nanofibers; (b) the typical high-resolution XPS spectra of O1s. | ||
The morphology and microstructure of the samples were investigated by TEM and HRTEM. For BiFeO3 nanoparticles, the images in Fig. 3a and b demonstrate that large-scale rust-red BiFeO3 nanoparticles (inset of Fig. 3a) possess uniform size and well monodisperse with a diameter of about 25 nm. They are identified as polycrystalline quasi hexahedron structure from SAED pattern (inset of Fig. 3b). Fig. 3c shows a typical SEM image of BT10 nanofibers. These light-brown BT10 nanofibers (inset of Fig. 3c) were as long as dozens of micrometers or even at the millimeter scale with the diameters of about 100–300 nm. They are aligned in random orientation because of the bending instability associated with the spinning jet. The magnified TEM image of BT10 nanofiber (Fig. 3d) clearly shows that a single nanofiber was compactly packed with nanoparticles, which agglomerated to form BiFeO3/TiO2 nanofibers. To better investigate the crystal structure and micro-composition of the TiO2 nanocomposite, HRTEM of as-obtained BT10 sample was performed and shown in Fig. 3e and f. The lattice fringes of the (110) planes with interplanar spacing of approximately 0.357 nm correspond to TiO2, whereas the fringes of the (024) planes with interplanar spacings of approximately 0.192 nm correspond to BiFeO3, indicating that TiO2 and BiFeO3 coexist in the microspheres. Obviously, BiFeO3 nanoparticles are surrounded by anatase-type TiO2 nanocrystals. The SAED pattern further confirms its polycrystalline property (inset of Fig. 3e).
The optical properties of samples were studied by UV-vis DRS, which is helpful for characterizing the optical absorption property and band gap energy of the semiconductor. In the study, the UV-vis diffuse reflectance spectra of the as-prepared samples were measured and converted into absorption spectra according to the Kubelka–Munk method. The corresponding band gaps are calculated from the plots of Eg = 1240/λ by extrapolating the linear portion of absorbance to the wavelength axis at absorbance = 0. As shown in Fig. 4, The TiO2 nanofibers exhibit the characteristic spectra of TiO2 with a steep absorption edge located at 380 nm (curve a). By fitting the plots, the band gap of the TiO2 nanofiber is evaluated to be about 3.2 eV. BiFeO3 nanoparticles display obvious absorption up to 600 nm (curve g), the corresponding bandgap is calculated to be 2.09 eV, which is in good agreement on the previously reported value.33,34 Hence, BiFeO3 has high absorption either in the UV range or in the visible light region. Meanwhile, the absorbance spectra reveal that all of these BiFeO3/TiO2 nanofibers possess absorption edges extending into the visible-light region, and the absorption edges show continuous red-shift with the increasing of BiFeO3 content.
The photocatalytic activities of the above samples on the degradation of MB under visible light irradiation were shown in Fig. 5. It could be seen that the pure TiO2 nanofibers exhibited poor photocatalytic activity, its photodegradation efficiency of MB only reached about 3% within 150 min under visible light irradiation. The activity of pure BiFeO3 sample is much higher than that of TiO2, its photodegradation efficiency of MB reached about 20% within 150 min. The BiFeO3/TiO2 heterojunctions showed better photocatalytic activity, the photodegradation efficiency of MB reached about 25% after 150 min of reaction for BT20 sample. In comparison, nearly 100% of MB is photocatalytically degraded by BT10 and BT5 within 150 min under visible light irradiation. Even the BT15 sample, only with a small loading amount of BiFeO3, still showed higher photocatalytic efficiency than that of pure TiO2 and BiFeO3 samples. Obviously, the photocatalytic activity of BiFeO3/TiO2 did not strictly increasing with the increasing of BiFeO3 content. The superior reactivity of the BiFeO3/TiO2 heterojunctions was observed at BT10 ([BiFeO3] :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TiO2] = 1
[TiO2] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9). The possible reason maybe when the molar ratio of BiFeO3 to TiO2 is below the critical ratio, less BiFeO3 decreased the visible-light absorption and correspondingly reduced the photocatalytic activity. When it is above the critical ratio, excessive BiFeO3 covered the active sites of TiO2 and hindered the electron transfer on the interfaces of BiFeO3/TiO2, correspondingly resulting in poor photocatalytic activity.
9). The possible reason maybe when the molar ratio of BiFeO3 to TiO2 is below the critical ratio, less BiFeO3 decreased the visible-light absorption and correspondingly reduced the photocatalytic activity. When it is above the critical ratio, excessive BiFeO3 covered the active sites of TiO2 and hindered the electron transfer on the interfaces of BiFeO3/TiO2, correspondingly resulting in poor photocatalytic activity.
 | ||
| Fig. 5 The visible-induced photocatalytical activity of (a) TiO2, (b) BiFeO3, (c) BT20, (d) BT15, (e) BT8, (f) BT5, (g) BT10. | ||
To understanding the heterojunction effect on the enhanced photocatalytic activity of BiFeO3/TiO2 heterostructures, the transient photocurrent responses of all samples (photocurrent (I)–time (t) response) were measured. Fig. 6 shows the typical I–t response curves for different samples with several on–off cycles of intermittent visible light irradiation. The photocurrent of the BiFeO3/TiO2 nanofibers are much higher than that of TiO2 except for BT20 sample, the current value of the undoped TiO2, BT5, BT8, BT10, BT15 and BT20 electrodes were 2.74, 16.85, 18.56, 7.72 and 1.75 μA cm−2, respectively. Obviously, the BT10 sample shows a highest photocurrent, which is about 6.77 times as high as those of the TiO2 electrode. The obvious enhancement of BiFeO3/TiO2 series samples in photocurrent indicated smaller recombination and more efficient separation of photogenerated electron–hole pairs at their interface.
 | ||
| Fig. 6 Photocurrent generation on the catalyst electrodes coated with TiO2 and BiFeO3/TiO2 samples; [Na2SO4] = 0.5 M; λ > 420 nm, continuously N2 purged. | ||
To further investigate the charge-transfer behaviors, we employed the EIS, which is a powerful technique to probe the charge transfer mechanisms at the photocatalyst/solution interface.35–37 In the Nyquist diagrams, the semicircle diameter of EIS is equal to surface charge-transfer resistance (Rct). For the photocatalytic reaction, a decrease in the radius of resistance always means a quicker charge transfer and a slower recombination of photoelectron and holes occurred, which is beneficial to relevant photocatalytic activity. From Fig. 7, we can see that the semicircle radius decreases in the order of BiFeO3 > TiO2> BT20 > BT15 > BT8 > BT5 > BT10. For TiO2 sample, a semicircle with Rct of 3500 Ω was obtained, which is much larger than the other samples, suggesting that the TiO2 nanofibers under visible light irradiation need to overcome a great energy barrier to take place photocatalytic reaction. For BiFeO3 nanoparticles, a semicircle with Rct is 2100 Ω, suggesting that it has less energy barrier than that of TiO2 under the same condition. For BiFeO3/TiO2 samples (BT10), it's semicircle with Rct was sharply reduced to 600 Ω, suggesting that the close contact of BiFeO3 with TiO2 was rather effective in suppression of the electron–hole recombination and acceleration of photocatalytic reaction. As a consequence, more long-lived photogenerated charges contribute to the photodegradation organic pollants.
The band structures of BiFeO3 and TiO2 were further estimated by the electronegativity of the oxide. The valence band (VB) and conduction band (CB) position of BiFeO3 and TiO2 at the point of zero charge can be calculated by the following empirical equation:38,39
| EVB = X − Ee + 0.5Eg | (1) |
| ECB = EVB − Eg | (2) |
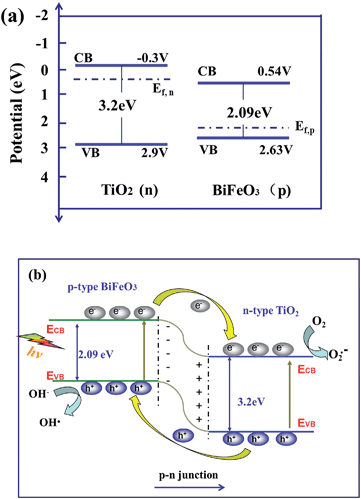 | ||
| Scheme 1 (a) the band energy schematic diagram of BiFeO3 and TiO2 before contace. (b) Postulate of the visible light-induced photo-degradation mechanism of BiFeO3/TiO2 nanofibers. | ||
In general, BiFeO3 is reported to be a p-type semiconductor,40,41 whose Fermi energy level lies close to the valence band and its band gap can be changed from 2.0 to 2.7 eV. Here, we will assume a value of 2.09 eV according to the UV-vis analysis. Undoped TiO2 is typically oxygen-deficit and thus is considered n-type with a band gap of 3.2 eV (for anatase)42,43 whose Fermi energy level lies close to the conduction band (CB) (see Scheme 1a). When TiO2 is coupled with BiFeO3 to form the p–n junction, the Fermi energy level of BiFeO3 and TiO2 tend to descend and rise up. Meanwhile, an inner electric field will be formed in the interface of p–n heterojunctions. At equilibrium, the inner electric field makes the p-type semiconductor region have a negative charge, while the n-type semiconductor region has a positive charge. As a result, the region of TiO2 is positively charged, and its CB position is more positive than that of BiFeO3.26,44,45 Based on the above analysis, a plausible energy level diagram for the BiFeO3/TiO2 heterostructures (see Scheme 1b) was constructed.
When the BiFeO3/TiO2 p–n heterojunction is radiated by visible light with the photon energy higher or equal to the band gap of p-type or n-type semiconductors, the photo-generated electrons can move to the CB of n-type semiconductors and the photo-generated holes to the VB of p-type semiconductors due to the formation of an inner electric field. Thus, the formation of the p–n heterojunction effectively hinders the recombination of photogenerated electron and hole and the photocatalytic efficiency is much enhanced.
For the practical applications, it is necessary to investigate the long-term stability of a photocatalyst during photocatalytic reaction. The conversions of MB obtained in five successive reaction cycles on the BiFeO3/TiO2 p–n junction nanofibers are shown in Fig. 8. The activity of BiFeO3/TiO2 catalysts decreased about 10% upon 5-recycling tests. So, The BiFeO3/TiO2 p–n junction nanofibers can be used as high-performance visible-light photocatalysts and for potential applications in environmental protection.
 | ||
| Fig. 8 Recyclability of the BiFeO3/TiO2 nanofibers for the degradation of MB under visible light irradiation. | ||
Conclusions
In conclusion, the novel BiFeO3/TiO2 p–n junction nanofibers have been prepared for the first time by hydrothermal process combined with electrospinning technique, which was successful to realize a close contact of p-type BiFeO3 nanoparticles with n-type TiO2 nanoparticles in the BiFeO3/TiO2 nanofibers, as evidenced by TEM and HRTEM observations. Such close contact was effective in suppression of the electron–hole recombination. The composite showed high visible light responding ability, excellent electron–hole mobility, and low electron–hole recombination, and therefore demonstrated superior photocatalytic activity and stability.Acknowledgements
The authors would like to acknowledge the financial support from the National Natural Science Foundation of China (Grant no. 51272185, 51172166 & 61205166), the National Program on key Basic Research Project (973 Grant no. 2012CB821404), and Subsidy of Large-scale Instrument & equipment of Wuhan University (Grant no. LF20130288)Notes and references
- V. A. Ganesh, H. K. Raut, A. S. Nair and S. Ramakrishna, J. Mater. Chem., 2011, 21, 16304–16322 RSC.
- Q. L. Yu, M. M. Ballari and H. J. H. Brouwers, Appl. Catal., B, 2010, 99, 58–65 CrossRef CAS PubMed.
- X. Chen, S. Shen, L. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503–6570 CrossRef CAS PubMed.
- A. Fujishima, X. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515–582 CrossRef CAS PubMed.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- D. Mitoraj and H. Kisch, Angew. Chem., Int. Ed., 2008, 47, 9975–9978 CrossRef CAS PubMed.
- S. In, A. Orlov, R. Berg, F. García, S. Pedrosa-Jimenez, M. S. Tikhov, D. S. Wright and R. M. Lambert, J. Am. Chem. Soc., 2007, 129, 13790–13791 CrossRef CAS PubMed.
- Y. Xie and X. J. Zhao, J. Mol. Catal. A: Chem., 2008, 285, 142–149 CrossRef CAS PubMed.
- F. Dong, W. R. Zhao and Z. B. Wu, Nanotechnology, 2008, 19, 365607 CrossRef PubMed.
- X. F. Wu, H. Y. Song, J. M. Yoon, Y. T. Yu and Y. F. Chen, Langmuir, 2009, 25, 6438–6447 CrossRef CAS PubMed.
- C. H. Kim, B. H. Kim and K. S. Yang, Synth. Met., 2011, 161, 1068–1072 CrossRef CAS PubMed.
- Z. Mesgari, M. Gharagozlou, A. Khosravi and K. Gharanjig, Appl. Catal., A, 2012, 411, 139–145 CrossRef PubMed.
- H. G. Yu, H. Irie, Y. Shimodaira, Y. Hosogi, Y. Kuroda, M. Miyauchi and K. Hashimoto, J. Phys. Chem. C, 2010, 114, 16481–16487 CAS.
- M. Hamadanian, A. Reisi-Vanani and A. Majedi, Appl. Surf. Sci., 2010, 256, 1837–1844 CrossRef CAS PubMed.
- G. Halasi, G. Schubert and F. Solymosi, J. Catal., 2012, 294, 199–206 CrossRef CAS PubMed.
- F. Kiriakidou, D. I. Kondarides and X. E. Verykios, Catal. Today, 1999, 54, 119–130 CrossRef CAS.
- J. B. Mu, B. Chen, M. Y. Zhang, Z. C. Guo, P. Zhang, Z. Y. Zhang, Y. Y. Sun, C. L. Shao and Y. C. Liu, ACS Appl. Mater. Interfaces, 2012, 4, 424–430 CAS.
- M. Y. Zhang, C. L. Shao, J. B. Mu, Z. Y. Zhang, Z. C. Guo, P. Zhang and Y. C. Liu, CrystEngComm, 2012, 14, 605–612 RSC.
- Y. P. Zhang, L. F. Fei, X. D. Jiang, C. X. Pan and Y. Wang, J. Am. Ceram. Soc., 2011, 94, 4157–4161 CrossRef CAS PubMed.
- D. P. Sun, J. Z. Yang and X. Wang, Nanoscale, 2010, 2, 287–292 RSC.
- Q. Y. Wang, X. C. Yang, D. Liu, L. Chi and J. W. Hou, Electrochim. Acta, 2012, 83, 140–145 CrossRef CAS PubMed.
- Y. M. Huo, Y. Jin and Y. Zhang, J. Mol. Catal. A: Chem., 2010, 331, 15–20 CrossRef CAS PubMed.
- U. A. Joshi, J. S. Jang, P. H. Borse and J. S. Lee, Appl. Phys. Lett., 2008, 92, 242106 CrossRef PubMed.
- M. Popa, D. Crespo, J. M. Calderon-Moreno, S. Preda and V. Fruth, J. Am. Ceram. Soc., 2007, 90, 2723–2727 CrossRef CAS PubMed.
- S. Li, Y. H. Lin, B. P. Zhang, J. F. Li and C. W. Nan, J. Appl. Phys., 2009, 105, 054310 CrossRef PubMed.
- Y. L. Zhang, A. M. Schultz, P. A. Salvador and G. S. Rohrer, J. Mater. Chem., 2011, 21, 4168–4417 RSC.
- M. Shang, W. Z. Wang, S. M. Sun, E. P. Gao, Z. J. Zhang, L. Zhang and R. O'Hayre, Nanoscale, 2013, 5, 5036–5042 RSC.
- H. G. Kim, P. H. Borse, J. S. Jang, C. W. Ahn, E. D. Jeong and J. S. Lee, Adv. Mater., 2011, 23, 2088–2092 CrossRef CAS PubMed.
- S. K. Choi, S. Kim, S. K. Lim and H. Park, J. Phys. Chem. C, 2010, 114, 16475–16480 CAS.
- Y. C. Yang, J. W.Wen, J. H. Wei, R. Xiong, J. Shi and C. X. Pan, ACS Appl. Mater. Interfaces, 2013, 5, 6201–6207 CAS.
- M. M. Bergshoef and G. J. Vancso, Adv. Mater., 1999, 11, 1362–1365 CrossRef CAS.
- H. Yoshimoto, Y. M. Shin, H. Terai and J. P. Vacanti, Biomaterials, 2003, 24, 2077–2082 CrossRef CAS.
- P. S. V. Mocherla, C. Karthik, R. Ubic, M. S. R. Rao and C. Sudakar, Appl. Phys. Lett., 2013, 103, 022910 CrossRef PubMed.
- U. A. Joshi, J. S. Jang, P. H. Borse and J. S. Lee, Appl. Phys. Lett., 2008, 92, 242106 CrossRef PubMed.
- X. Wang, Q. Xu, M. R. Li, S. Shen, X. L. Wang, Y. C. Wang, Z. C. Feng, J. Y. Shi, H. X. Han and C. Li, Angew. Chem., Int. Ed., 2012, 51, 13089–13092 CrossRef CAS PubMed.
- S. J. Yu, H. J. Yun, Y. H. Kim and J. Yi, Appl. Catal., B, 2014, 144, 893–899 CrossRef CAS PubMed.
- N. Lakshminarasimhan, A. D. Bokare and W. Choi, J. Phys. Chem. C, 2012, 116, 17531–17539 CAS.
- Y. I. Kim, S. J. Atherton, E. S. Brigham and T. E. Mallouk, J. Phys. Chem. C, 1993, 97, 11802–11806 CrossRef CAS.
- G. Dai, J. Yu and G. Liu, J. Phys. Chem. C, 2011, 115, 7339–7346 CAS.
- N. Maso and A. R. West, Chem. Mater., 2012, 24, 2027–2032 CrossRef.
- T. R. Paudel, S. S. Jaswal and E. Y. Tsymbal, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 104409 CrossRef.
- K. Ishibashi, A. Fujishima, T. Watanabe and K. Hashimoto, Electrochem. Commun., 2000, 2, 207–210 CrossRef CAS.
- J. G. Yu, Q. J. Xiang and M. H. Zhou, Appl. Catal., B, 2009, 90, 595–602 CrossRef CAS PubMed.
- T. P. Xie, L. J. Xu, C. L. Liu, J. Yang and M. Wang, Dalton Trans., 2014, 43, 2211–2220 RSC.
- D. F. Hou, X. L. Hu, P. Hu, W. Zhang, M. F. Zhang and Y. H. Huang, Nanoscale, 2013, 5, 9764–9772 RSC.
| This journal is © The Royal Society of Chemistry 2014 |




