Copper nanoparticle-catalyzed borylation of alkyl bromides with an organodiboron compound†
Ju Hyun Kim and
Young Keun Chung*
Department of Chemistry, College of Natural Science, Seoul National University, Seoul, Korea. E-mail: ykchung@snu.ac.kr; Fax: +82-2-889-0310
First published on 19th August 2014
Abstract
Borylation of primary and secondary alkyl bromides with bis(pinacolato)diboron was carried out in the presence of commercially available copper nanoparticles as catalysts. The reaction went to completion in the absence of phosphines at room temperature. The catalytic system showed high activity, broad substrate scope, and good functional group tolerance.
The Suzuki–Miyaura cross-coupling reaction, catalyzed by a palladium(0) complex, and where the coupling partners are a boronic acid and a halide, shared the Nobel Prize for Chemistry 2010 because it was one of the most significant methodologies for the synthesis of biaryl compounds.1 Over the past decades, the synthetically useful organoboron-containing compounds have been actively developed.2 Recently, the transition metal-catalyzed borylation of the C–H bond has attracted much attention as an alternative method for the synthesis of the C–B bond.3 Among the transition metal–catalysts used for borylation reactions, palladium, nickel, and copper complexes are by far the most widely studied, mainly because of their extensive applicable scope, and compatibility with many functional groups.4–6 However, most of the catalytic systems involve the use of toxic ligands such as phosphine compounds. Therefore, there is a need for the development of environment-friendly and economically more convenient catalytic systems. In this regard, the use of copper-based catalysts5 is particularly attractive because they are inexpensive, readily available, and environmentally friendly. The use of an inexpensive Cu catalyst for the borylation of alkyl halides at room temperature was reported by Liu and Ito et al.6,7 Recently, the ligand-free copper-catalyzed borylation of benzyl halides with bis(pinacolato)diboron in DMF at 80 °C was reported by Yan.5c Previous studies on the copper-catalyzed cross-coupling reactions5,6 provided important motives to use copper nanoparticles (Cu NPs) as catalysts in the coupling reactions of alkyl halides with diboron reagents. It has been recently reported8 that copper(II) oxide nanoparticles (CuO NPs) supported on magnesia exhibits complete chemoselectivity towards the monoborylation of alkynes in the presence of alkenes and triphenylphosphine. However, until now, the catalysis of such transformations by Cu NPs has not been reported yet. Therefore, we decided to study the use of Cu NPs in the borylation of alkyl halides with alkoxy diboron compounds. It was found that Cu NPs were quite effective catalysts in the borylation of primary and secondary alkyl bromides with bis(pinacolato)diboron. Herein we communicate our preliminary results. Cu NPs could efficiently catalyze the cross-coupling of alkylboronate with nonactivated alkyl bromides in the absence of phosphines at room temperatures.
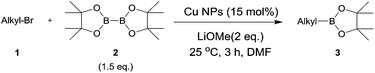
The reaction of 1-bromooctane (1a) with bis(pinacolato)diboron (2) to produce pinacol alkylboronate (3a) was chosen as a model reaction in Scheme 1. We carried out the optimization of the catalyst system. The same catalyst that we have studied our previous report9 was used. The results are summarized in Table 1. The treatment of 1a with 2 in the presence of 15 mol% of Cu NPs and 2 equiv. of LiOtBu in DMF at 25 °C for 18 h was found to produce the corresponding alkylboronate 3a in a good yield (99%, entry 1). The reaction time could be decreased to 3 h without any loss in the yield (99%, entry 2). To improve the yield, different bases such as LiOMe, K2CO3, NaOH, and NaOMe were studied (entries 3–6). It was observed that the base LiOMe afforded good yields of the desired product (90%). However, other bases such as K2CO3, NaOH, and NaOMe were not effective (yields: 32–69%). LiOtBu was found to be the best base giving a yield of 99% (entry 2), but it was effective only for normal alkyl bromides. Thus, we chose LiOMe as the base in our reaction. The effect of various solvents on the reaction system was also examined (entries 7–9). It was observed that solvents, such as THF, toluene, and MeCN, were proven to be inefficient for this transformation, but the reasons for this are not very clear. DMF was found to give the best results and was used for further studies. When wet DMF was used as a solvent with a reaction time of 3 h, the yield decreased to ca. 80%. Liu et al.6 reported that the addition of 4 equivalents of water reduced the yield from 91% to 77%. Thus, the reaction became slow in the presence of water. Interestingly, in our case, the yield recovered to 91% for a reaction time of 6 h. The necessity of Cu NPs in the reaction was confirmed by the observation that no reaction was observed in their absence (entry 10). We also examined other metal compounds such as Pd(OAc)2, NiCl2, and CuO NPs as a catalyst under the optimized reaction conditions (entries 11–13). In the presence of Pd(OAc)2, no reaction was observed. However, in the presence of NiCl2 and CuO NPs, the formation of 3a was observed in 3% and 31% yields, respectively. Although our previous study9 showed that CuO NPs were also an active catalyst in the C–C coupling reaction in the presence of Grignard reagents, the borylation reaction using CuO NPs was sluggish. Among the catalysts studied, no other catalysts afforded a higher yield than Cu NPs used. The possibility of contamination by palladium or nickel in the catalytic reaction was eliminated.10
| Entry | Cat. | Cat. (mol%) | Base (2 eq.) | Solvent (1 mL) | Time (h) | Yielda (%) |
|---|---|---|---|---|---|---|
| a Determined by GC analysis using 1,3,5-trimethylbenzene as an internal standard. | ||||||
| 1 | Cu NP | 15 | LiOt-Bu | DMF | 18 | 99 |
| 2 | Cu NP | 15 | LiOt-Bu | DMF | 3 | 99 |
| 3 | Cu NP | 15 | LiOMe | DMF | 3 | 90 |
| 4 | Cu NP | 15 | NaOMe | DMF | 3 | 32 |
| 5 | Cu NP | 15 | K2CO3 | DMF | 3 | 43 |
| 6 | Cu NP | 15 | NaOH | DMF | 3 | 69 |
| 7 | Cu NP | 15 | LiOMe | THF | 3 | 0 |
| 8 | Cu NP | 15 | LiOMe | Toluene | 3 | 0 |
| 9 | Cu NP | 15 | LiOMe | MeCN | 3 | 0 |
| 10 | No cat. | LiOt-Bu | DMF | 3 | 0 | |
| 11 | Pd(OAc)2 | 15 | LiOMe | DMF | 3 | 0 |
| 12 | NiCl2 | 15 | LiOMe | DMF | 3 | 3 |
| 13 | CuO NP | 15 | LiOMe | DMF | 3 | 31 |
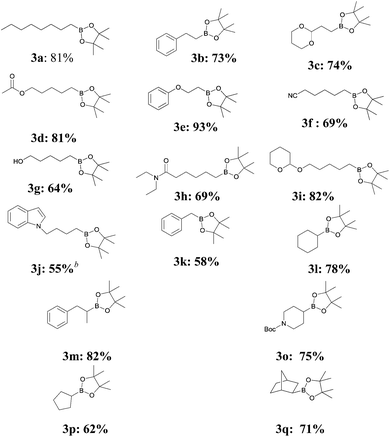
With the optimized conditions in hand, we next investigated the substrate scope of the borylation of the alkyl bromides using 2 (Table 2).
The yield ranged from 55% to 93%. The presence of functional groups (3a–3k), such as acetal, ester, hydroxy, amide, and cyano groups, and an ether linkage in the starting alkyl bromides 1 did not interfere with the outcome of the borylation with 1. In particular, it is notable that even without a protecting group, the borylation reaction proceeds in the presence of a hydroxyl group (3g).6 Compounds containing heterocyclic arenes, such as pyran and indole (3i–3j) are good substrates for the borylation. Interestingly, (2-bromoethoxy)benzene, which is known to undergo a C–O bond cleavage in the reaction with a Grignard reagent in the presence of Cu NPs,9 afforded a high yield (93%) of the borylation product (entry 4). Compared to a homogeneous copper system (CuI and PPh3),7 the Cu NP system showed a higher activity for some substrates having functional groups such as a hydroxyl, or an amide, but showed lesser activity for other substrates having nitrile, indole, or benzyl groups. However, it seems that the overall reactivity of the Cu NP system seemed to be similar to that of the homogeneous copper system.
We also studied the borylation of secondary alkyl bromides under our optimized reaction conditions (3l–3q). For the borylation of exo-2-bromonorbornane, an overall retention of configuration was observed (3q).6 Unactivated secondary alkyl halides could be borylated with good yields (62–82%). Cyclic and acyclic secondary bromides were easily borylated and a protected amine was also a good substrate. For cyclohexyl bromide, the yield was 78% at 25 °C for 3 h. This yield was higher than that (60%) observed in the presence of CuI and PPh3 at 25 °C for 24 h by Liu et al.6 although they observed a 79% yield at a higher reaction temperature (37 °C). Thus, our catalytic system was highly effective for the borylation of primary and secondary alkyl bromides, but primary alkyl chlorides and tertiary alkyl halides were found to be inert under our reaction conditions.
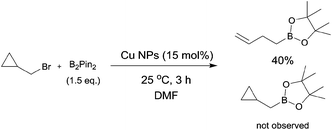 | (1) |
To validate the reaction mechanism further, we carried out an experiment (eqn (1)). When the borylation of cyclopropylmethyl bromide was carried out in the presence of Cu NPs, 3-butenylboronate was isolated in 40% yield and no simple boryl substitution product was observed. In contrast to the case of Cu NP-catalyzed coupling reaction in the presence of a Grignard reagent,9 the observation of the ring-opening product provided an evidence for the formation of a radical intermediate, which suggests that the reaction probably proceeds via a radical pathway.
Conclusions
We have developed a remarkably simple and general Cu NP-catalyzed borylation reaction under phosphine-free conditions. The Cu NPs used in this study are commercially available, and the catalytic system does not require any pretreatment or preformed supports and ligands. The catalytic system is quite effective for the borylation of unactivated primary and secondary alkyl bromides with organodiboron compounds. In view of the broad substrate scope, functional group tolerance, high reaction efficiencies, and high product yields, the Cu NP-catalyzed cross-coupling reaction can be expected to find wide synthetic applications. Further mechanistic studies are in progress.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) (2007-0093864 and 2014-011165). JHK thanks the Brain Korea 21 plus fellowships.Notes and references
- (a) F.-S. Han, Chem. Soc. Rev., 2013, 42, 5270 RSC; (b) F. Bellina, A. Carpita and R. Rossi, Synthesis, 2004, 2419 CAS; (c) S. Kotha, K. Lahiri and D. Kashinath, Tetrahedron, 2002, 58, 9633 CrossRef CAS.
- (a) Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, New York, 2004; Search PubMed; (b) Boronic Acids, ed. D. G. Hall, Wiley-VCH, Weinheim, Germany, 2005; Search PubMed; (c) G. A. Molander and N. Ellis, Acc. Chem. Res., 2007, 40, 275 CrossRef CAS PubMed; (d) R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461 CrossRef CAS PubMed.
- (a) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (b) C. Bae, Alkane C-H Activation by Single-Site Metal Catalysis in Catalysis by Metal Complexes 38, ed. P. J. Pérez, Springer Science + Business Media Dordrecht, 2012, ch. 3, pp. 73–111 Search PubMed; (c) I. A. I. Mikhalid, J. H. Barnard, T. B. Marder, J. M. Murphy and J. F. Hartwig, Chem. Rev., 2010, 110, 890 CrossRef PubMed; (d) J. F. Hartiwg, Acc. Chem. Res., 2012, 45, 864 CrossRef PubMed; (e) A. G. Green, P. Liu, C. A. Merlic and K. N. Houk, J. Am. Chem. Soc., 2014, 136, 4575 CrossRef CAS PubMed.
- For Pd-catalyzed reactions, see: (a) T. Ishiyama, M. Murata and N. Miyaura, J. Org. Chem., 1995, 60, 7508 CrossRef CAS; (b) T. Ishiyama, M. Murata and N. Miyaura, Tetrahedron, 2001, 57, 9813 CrossRef CAS; (c) K. L. Billingsley, T. E. Barder and S. L. Buchwald, Angew. Chem., Int. Ed., 2007, 46, 5359 CrossRef CAS PubMed; (d) S. Kawamorita, H. Ohmiya, T. Iwai and M. Sawamura, Angew. Chem., Int. Ed., 2011, 50, 8363 CrossRef CAS PubMed; (e) C. M. So and F. Y. Kwong, Chem. Soc. Rev., 2011, 40, 4963 RSC; (f) For Ni-catalyzed reactions, see: G. A. Molander, L. N. Cavalanti and C. García-García, J. Org. Chem., 2013, 78, 6427 CrossRef CAS PubMed and references therein; (g) P. Leowanawat, N. Zhang and V. Percec, J. Org. Chem., 2012, 77, 1018 CrossRef CAS PubMed; (h) T. Yamamoto, T. Morita, J. Takagi and T. Yamakawa, Org. Lett., 2011, 13, 5766 CrossRef CAS PubMed; (i) D. A. Wilson, C. J. Wilson, C. Moldoveanu, A.-M. Resmerita, P. Corcoran, L. M. Hoang, B. M. Rosen and V. Percec, J. Am. Chem. Soc., 2010, 132, 1800 CrossRef CAS PubMed.
- (a) W. Zhu and D. Ma, Org. Lett., 2006, 8, 261 CrossRef CAS PubMed; (b) C. Kleeberg, L. Dang, Z. Lin and T. B. Marder, Angew. Chem., Int. Ed., 2009, 48, 5350 CrossRef CAS PubMed; (c) G. Yan, M. Yang and J. Yu, Lett. Org. Chem., 2012, 9, 71 CrossRef CAS; (d) C.-T. Yang, Z.-Q. Zhang, Y.-C. Liu and L. Liu, Angew. Chem., Int. Ed., 2011, 50, 3904 CrossRef CAS PubMed.
- C.-T. Yang, Z.-Q. Zhang, H. Tajuddin, C.-C. Wu, J. Liang, J.-H. Liu, Y. Fu, M. Czyzewska, P. G. Steel, T. B. Marder and L. Liu, Angew. Chem., Int. Ed., 2012, 51, 528 CrossRef CAS PubMed.
- H. Ito and K. Kubota, Org. Lett., 2012, 14, 890 CrossRef CAS PubMed.
- A. Grirrane, A. Corma and H. Garcia, Chem.–Eur. J., 2011, 17, 2467 CrossRef CAS PubMed.
- J. H. Kim and Y. K. Chung, Chem. Commun., 2013, 49, 11101 RSC.
- I. Thomé, A. Nijs and C. Bolm, Chem. Soc. Rev., 2012, 41, 979 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05999a |
| This journal is © The Royal Society of Chemistry 2014 |