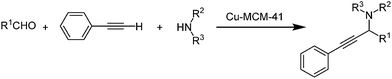
Copper modified spherical MCM-41 nano particles: an efficient catalyst for the three-component coupling of aldehydes, amines and alkynes in solvent-free conditions
Mohammad Abdollahi-Alibeik* and
Ali Moaddeli
Department of Chemistry, Yazd University, Yazd 89195-741, Iran. E-mail: abdollahi@yazd.ac.ir; moabdollahi@gmail.com; Fax: +98-351-8210644; Tel: +98-351-8122659
First published on 13th August 2014
Abstract
Cu-MCM-41 nano particles have been shown to effectively catalyze the multi-component synthesis of propargylamines by the reaction of aldehydes, amines, and alkynes (A3 coupling) effectively at 100 °C under solvent-free conditions. Spherical Cu-MCM-41 nanoparticles were prepared by a simple method and characterized by XRD, TEM, SEM and FT-IR techniques. Cu-MCM-41 with a Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratio of 20
Cu molar ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 shows the best catalytic activity. The catalyst was recovered by simple filtration and reused for several cycles without considerable loss of activity.
1 shows the best catalytic activity. The catalyst was recovered by simple filtration and reused for several cycles without considerable loss of activity.
1. Introduction
Propargylamines represent an important class of compounds, which are found in various biologically active naturally existing materials. These compounds have also been used as precursors in the synthesis of heterocyclic compounds such as quinolines,1 phenanthrolines,2 pyrroles,3 pyrrolidines,4 indolizines5 and oxazolidinones.6 Propargylamines can be utilized as versatile and key synthetic intermediates for the preparation of several natural products and bioactive compounds.7,8In general, propargylamines have been constructed through various methods like nucleophilic addition of in situ generated metal acetylides to imines and enamines,9,10 amination of propargylic electrophiles (halides, propargylic phosphates or propargylic triflates)11 and highly efficient three-component coupling reaction through C–H activation (Mannich condensation).12 Among these synthetic strategies, three-component Mannich condensation of terminal alkynes, aldehydes, and amines is more efficient and certainly the most popular method due to building complex structures from simple starting materials which are available commercially. In addition, this reaction is performed in an environmentally compatible fashion through one-pot multi-component reaction (MCRs) in a catalytic process and also atom-economical approach.
Various catalyst have been developed for this type of reaction, such as CuSBA-15, iridium,13 gold,14 nickel,15 iron16 and indium,17 and also various solvents such as toluene,18 acetonitrile19 and chloroforme20 have been used. However, many of these methods suffer from at least one of disadvantages, such as the use of toxic solvent, and the use of expensive and non-reusable catalyst.
Recently heterogenous catalysts have been considered by chemists due to the unique advantages, such as simple handling and storage, easy separation and regenerability.21 MCM-41 as one of the members of mesoporous silicas, has been applied as heterogeneous catalyst and support in many organic processes.22 MCM-41 possesses a high surface area and uniform tubular channels (2D-hexagonal structure) with tunable pore diameters in the range of 2–10 nm.23–25 These properties provide good transportation channels for reactants to access active centers and for products to move out. Due to the low catalytic activities of MCM-41, modified MCM-41 has attracted considerable attention.26–30 Transition metal (such as V,31 Fe,32 Cu,33 Mn,34 Co,35 Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 and Mo
36 and Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37) modified MCM-41 has emerged as useful heterogeneous catalysts due to their versatile applications in synthesis and catalysis.
37) modified MCM-41 has emerged as useful heterogeneous catalysts due to their versatile applications in synthesis and catalysis.
C–C bond formation reaction is one of the most important processes in chemistry because they provide key steps in building more complex molecules from simple precursors. Transition metal catalyzed C–H bond activations and subsequent C–C bond formations have attracted great interest in recent years.38
Copper is an efficient, cheap and non-toxic ingredient in many heterogeneous and homogeneous catalysts particularly for the synthesis of many organic compounds especially through C–H bond activation for C–C bond formation.39
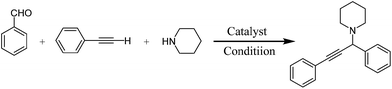
To the best of our knowledge, there are no examples of C–H bond activation using copper-based mesoporous silica catalysts. We expect that copper-modified mesoporous silica show favorable activity in this type of reactions (Scheme 1). It is considerable that in the presence study a novel method was developed for the preparation of Cu-MCM-41 nanoparticles under mild condition and without using hydrothermal method.
2. Experimental
2.1 Material and methods
All chemicals were commercial products. All reactions were monitored by TLC (thin layer chromatography) and all yields refer to isolated products. 1H and 13C NMR spectra were recorded in CDCl3 on a Bruker DRX-400 AVANCE (400 MHz for 1H and 100 MHz for13C) spectrometer. Infrared spectra of the catalysts and reaction products were recorded on a Bruker FT-IR Equinox 55 instrument spectrophotometer in KBr disks. XRD patterns were recorded on a Bruker D8 ADVANCE X-ray diffractometer using nickel filtered Cu Kα radiation (λ = 1.5406 Å). Scanning electron micrograms (SEM) was obtained using KYKY-EM3200 Instrument. Transmittance electron microscopy was performed with Zeiss-EM10C at 80 KV. Potentiometric data was collected using pH/mV meter, AZ model 86502-pH/ORP.2.2 Preparation of Cu-MCM-41 nanoparticles
The synthesis of nano-sized Cu-MCM-41 was carried out by method of direct insertion of copper ion in sol–gel preparation step at room temperature (RT) using tetraethylorthosilicate (TEOS) as the Si source, cetyltrimethylammonium bromide (CTAB) as the template, ammonia as the pH control agent and Cu(OAc)2·H2O as the copper source with the gel composition (molar ratio) of SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(OAc)2·H2O
Cu(OAc)2·H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CTAB
CTAB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH4OH
NH4OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1.00
H2O = 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.050
0.050![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.127
0.127![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.623
0.623![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 508.
508.
Cetyltrimethylammonium bromide (1.04 g) was dissolved in deionized water (200 mL) under stirring, and then temperature was adjusted to 60 °C for 15 min. To this solution, tetraethylorthosilicate (5 mL) was added dropwise and then a solution of Cu(OAc)2.H2O (appropriate amount of copper precursor in 5 mL of deionized water) was added dropwise under vigorous stirring while, the temperature was maintained at 60 °C. The reaction mixture was allowed to cool to RT and then pH of the solution was adjusted to 10.5 by adding 25 wt% ammonia solution. The mixture was stirred for 12 h at RT. The gel was recovered by centrifuging and washed with ethanol (3 × 5 mL) and deionized water (3 × 10 mL). The obtained solid was dried at 120 °C for 2 h and calcined in air at 550 °C for 4 h. The obtained samples with Si : Cu molar ratio of 10, 20 and 30 were denoted as 10-CM, 20-CM and 30-CM respectively.
2.3 General procedure for the synthesis of propargylamine derivatives
Aromatic/aliphatic aldehydes 1a–n (1 mmol), secondary amine (1.1 mmol), phenylacetylene (1.2 mmol) and catalyst (40 mg) were added into a 5 mL round bottom flask and were stirred at 100 °C. The progress of the reaction was monitored by TLC. After completion of the reaction, ethanol (2 mL) was added to the reaction mixture and the solution was centrifuged at 3000 rpm for 2 min. Finally, the excess of solvent was removed under reduced pressure to give the corresponding product (4a–n). Further purification was achieved by thin layer chromatography on silica gel using n-hexane/ethylacetate.2.4 Physical and spectroscopic data for selected compounds
3. Results and discussions
3.1 The catalyst characterization
Fig. 1 represents the results of scanning electron micrograms (SEM) in order to investigate the particle size and morphology of the catalysts. The SEM of Cu-MCM-41 shows spherical nanoparticles with sizes of <100 nm.The FT-IR absorption spectra of pure silica MCM-41 and Cu-incorporated MCM-41 samples with different loading amounts of copper are shown in Fig. 2. In the range of 400–1600 cm−1, three peaks at ∼465 cm−1, ∼800 cm−1 and ∼1090 cm−1 are corresponding to the rocking, bending (or symmetric stretching), and asymmetric stretching of the inter-tetrahedral oxygen atoms in SiO2 of Cu-MCM-41, respectively and also the peak at 966 cm−1 is assigned to the silanol group. The vibration absorption band at 1089 cm−1 is assigned to ν(Si–O–Si) and a slight red-shift to the lower frequencies is observed in Cu-MCM-41 samples. In general, this shift of the absorption peaks toward the lower wavenumbers is considered an indication of Cu incorporation into the framework of silica tetrahedra.
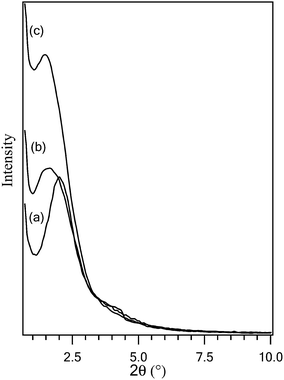
The low angle XRD patterns of Cu-MCM-41 samples with Si : Cu molar ratio of 10, 20 and 30 are shown in Fig. 3. The intensity of the main peak of Cu-MCM-41 samples was decreased with increasing in copper content of the catalysts. These changes are due to the decrease in the long-range order of the hexagonal meso-structure of MCM-41 by the incorporation of copper into the hexagonal channels of MCM-41. The main peaks of samples is observed in 2θ = 1.98, 1.55, 1.45 for 10-CM, 20-CM and 30-CM, respectively. An increase in the d-values (a decrease in 2θ based on the Bragg's equation) and unitcell parameters are also observed on incorporation of copper and they increase with increasing copper content. These results are probably due to the partial substitution of the structural Si4+ by the Cu2+ ion, resulting in collapse of the hexagonal structure of MCM-41 and the increase in unit-cell parameters on Cu incorporation is probably due to the larger size of Cu2+ compared to Si4+.
As shown in Fig. 4a, high angle XRD pattern of 10-CM show slightly CuO in tenorite phase while 20-CM and 30-CM with lower Cu content (Fig 4b) lack this phase. This fact show high dispersion of copper ions in the MCM-41 framework and confirms absence of distinct phase for CuO especially for 20-CM.
Mesostructure of the 20-CM sample was further studied by TEM, as shown in Fig. 5. Porosity of the sample is clear and pores size is observed in the range of 2.5–3 nm. No copper oxide nanoparticles are observed in TEM image, suggesting that copper is completely dispersed in MCM-41 framework. Partial disordered mesoporous system is related to the incorporation of Cu into the MCM-41 framework that confirms by XRD data.
The catalyst acidity characters, including the acidic strength and the total number of acid sites were determined by potentiometric titration. According to this method, the initial electrode potential (Ei) indicates the maximum acid strength of the surface sites.40 Therefore, a suspension of the catalyst in acetonitrile was potentiometrically titrated with a solution of 0.02 M n-butylamine in acetonitrile. As shown in Fig. 6, 20-CM displays higher acid strength than the other samples.
In order to obtain a clear distinction between Lewis and Brønsted acid sites, FT-IR analysis of pyridine adsorbed on the catalyst surface were carried out and results are displayed in Fig. 7. The FT-IR spectrum of pyridine adsorbed 20-CM before heat treatment (Fig. 7b) shows the contribution of pyridine adducts in the region of 1400–1650 cm−1. In this spectrum, the peaks at 1448 and 1598 cm−1 are attributed to pyridine bonded Lewis acid sites of the 20-CM. The weak peak at 1543 cm−1 assigned to Brønsted acid sites (corresponds to protonation of pyridine on Brønsted acid sites) is so hard to find in current zoom. The weak peak at 1491 cm−1 is attributed to the combination of pyridine bonded to Lewis and Brønsted acid sites. As shown in Fig. 7c–g, with increasing in temperature, characteristic peaks of Lewis acid sites are still remained at 1448 and 1599 cm−1. These results show that Lewis acidity of the catalyst is stronger than its Brønsted acidity.23
 | ||
| Fig. 7 FT-IR spectra of (a) 20-CM, (b) pyridine adsorbed 20-CM at ambient temperature and pyridine adsorbed 20-CM heated at (c) 100 °C, (d) 200 °C, (e) 300 °C, (f) 400 °C and (g) 500 °C. | ||
3.2 Catalytic activity of Cu-MCM-41
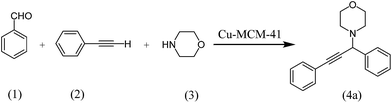
To testify the catalytic activity, synthesis of propargylamines through A3 coupling of aldehyde, amines and phenylacetylene is selected to represent activity of the prepared catalysts.In order to optimize the reaction conditions, initially, the reaction of benzaldehyde, phenylacetylene and morpholine was selected as model reaction (Scheme 2).
The reaction was optimized for the various parameters such as temperature, solvent and catalyst loading. To investigate the effect of reaction temperature, the reaction was performed initially in solvent free condition at various temperature (Table 1 entries 1–5). The best result was obtained at 100 °C. Increasing in the reaction temperature up to 120 °C led to a decrease in the yield of desired product due to the increasing in by-products and emission of the volatile precursors from the reaction media.
| Entry | Catalyst | Temp. (°C) | Catalyst amount (mg) | Cu contentb (mol%) | Solvent | Time (min) | Yield (%) |
|---|---|---|---|---|---|---|---|
| a All reactions were carried out with benzaldehyde (1 mmol), morpholine (1.1 mmol), phenylacetylene (1.2 mmol) and Cu-MCM-41 as catalyst.b Copper content of the catalyst was measured by atomic absorption instrumentation. | |||||||
| 1 | 20-CM | 80 | 40 | 2.9 | — | 180 | 60 |
| 2 | 20-CM | 90 | 40 | 2.9 | — | 180 | 80 |
| 3 | 20-CM | 100 | 40 | 2.9 | — | 180 | 85 |
| 4 | 20-CM | 110 | 40 | 2.9 | — | 150 | 79 |
| 5 | 20-CM | 120 | 40 | 2.9 | — | 150 | 74 |
| 6 | 20-CM | 100 | 20 | 1.5 | — | 180 | 65 |
| 7 | 20-CM | 100 | 30 | 2 | — | 180 | 72 |
| 8 | 20-CM | 100 | 50 | 3.6 | — | 180 | 80 |
| 9 | 10-CM | 100 | 40 | 5.8 | — | 180 | 80 |
| 10 | 30-CM | 100 | 40 | 1.9 | — | 180 | 68 |
| 11 | 20-CM | 100 | 100 | 7.3 | PhCH3 | 360 | 85 |
| 12 | 20-CM | Reflux | 100 | 7.3 | CH3CN | 360 | 50 |
| 13 | 20-CM | Reflux | 100 | 7.3 | THF | 24 h | 60 |
To optimize the catalyst amount, the model reaction was performed in the presence of various amount of the catalyst and according to the obtained results (Table 1, entries 6–10) 40 mg of the catalyst was chosen as the best catalyst amount. The effect of solvent was also investigated by performing the model reaction in the presence of 40 mg catalyst in various solvents (Table 1, entries 11–13). The model reaction in the presence of toluene as solvent showed convenient yield but it suffered from high loading of catalyst, long reaction time and toxic solvent (Table 1, entry 11).
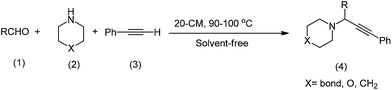
Thereafter, the above optimized reaction conditions were explored for the synthesis of propargylamine derivatives (Scheme 3) and the results are summarized in Table 2. As exemplified in Table 2, this protocol is rather general for a wide variety of electron-rich as well as electron-deficient aromatic aldehydes and also various secondary amines. Although a regular trend is not observed base on the activity of benzaldehyde derivatives but results in Table 2 show that pyrolidine have the most activity among the applied amines. This procedure is also convenient for aliphatic aldehydes (Table 2, entry j).
| Entry | Substrate (1) | Substrate (2) | Product (4) | Timeb (min) | Yieldc (%) |
|---|---|---|---|---|---|
| a Reaction conditions: aldehyde (1 mmol), phenylacetylene (1.2 mmol), amine (1.1 mmol), Cu-MCM-41 (40 mg) and temperature (90–100 °C).b Reactions time is based on the consumption of aldehyde monitored by TLC.c Isolated yield. | |||||
| a |  |
 |
 |
180 | 85 |
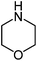
| b | 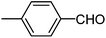 |
 |
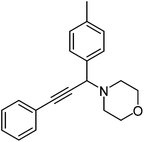 |
210 | 80 |
| c | 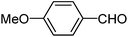 |
 |
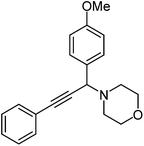 |
240 | 92 |
| d | 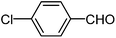 |
 |
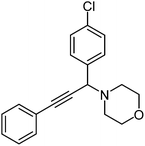 |
210 | 80 |
| e | 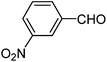 |
 |
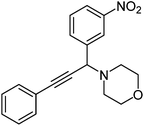 |
120 | 80 |
| f |  |
 |
 |
180 | 85 |
| g |  |
 |
 |
90 | 93 |
| h |  |
 |
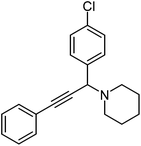 |
120 | 85 |
| i | 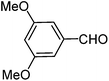 |
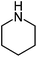 |
 |
90 | 90 |
| j | 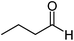 |
 |
 |
45 | 88 |
| k |  |
 |
 |
30 | 85 |
| l |  |
 |
 |
35 | 90 |
| m |  |
 |
 |
45 | 92 |
| N |  |
 |
 |
20 | 75 |
We perform a comparative study of the reactivity of Cu-MCM-41 with other reported heterogeneous Cu-based catalyst for the synthesis of propargylamines through A3 coupling reaction of benzaldehyde, piperidine, and phenylacetylene using Cu-based catalysts (Table 3) (Scheme 4). With an overall look at Table 3, we can say that our method is comparable with other catalytic systems in term of yield and reaction time. In addition to this, moderate reaction temperature, lack of requirement for an inert atmosphere, and absence of solvent are some obvious benefits with respect to the other methods.
Reusability of the catalyst was investigated in the model reaction under the optimized reaction conditions. The catalyst was separated from the model reaction and reused two times with moderate loss of the catalytic activity (Table 4). This result may be due to blockage of active sites of the catalyst and/or partial leaching of copper from the catalyst. Enhancement in the activity after calcination of recovered catalyst from cycle 2 confirms partial blockage of active sites of the catalyst (Table 4, cycle 3).
4. Conclusion
We introduced a new method for the synthesis of copper modified MCM-41 at room temperature without using hydrothermal condition. We found that copper content have large effect on the unit cell parameter and catalytic properties of Cu-MCM-41. Cu-MCM-41 materials with different Si–Cu molar ratio were characterized by XRD, FT-IR, SEM, TEM, pyridine absorption and potentiometric titration, and according to the characterization and optimization experiments, Cu-MCM-41 with Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu molar ratio of 20
Cu molar ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 shows the best catalytic activity. The catalyst has been applied, for the first time, as a heterogeneous and reusable catalyst in A3 coupling reaction of aldehydes, amines and phenylacetylene, a facile method for the synthesis of propargylamines under solvent-free condition. Cu-MCM-41 is able to catalyze this reaction efficiently without using base, inert atmosphere and solvent as compared to the other methods.
1 shows the best catalytic activity. The catalyst has been applied, for the first time, as a heterogeneous and reusable catalyst in A3 coupling reaction of aldehydes, amines and phenylacetylene, a facile method for the synthesis of propargylamines under solvent-free condition. Cu-MCM-41 is able to catalyze this reaction efficiently without using base, inert atmosphere and solvent as compared to the other methods.
In conclusion, an environmentally benign, economical, practical, and efficient process for the synthesis of propargylamine derivatives was improved through three-component coupling of various aldehydes, amines and phenylacetylene by using copper modified MCM-41 as a reusable and recoverable catalyst.
Acknowledgements
We are thankful to the Yazd University Research Council for partial support of this work.References
- F. Xiao, Y. Chen, Y. Liu and J. Wang, Tetrahedron, 2008, 64, 2755–2761 CrossRef CAS PubMed.
- D. Shibata, E. Okada, J. Molette and M. Médebielle, Tetrahedron Lett., 2008, 49, 7161–7164 CrossRef CAS PubMed.
- Y. Yamamoto, H. Hayashi, T. Saigoku and H. Nishiyama, J. Am. Chem. Soc., 2005, 127, 10804–10805 CrossRef CAS PubMed.
- D. F. Harvey and D. M. Sigano, J. Org. Chem., 1996, 61, 2268–2272 CrossRef CAS.
- B. Yan and Y. Liu, Org. Lett., 2007, 9, 4323–4326 CrossRef CAS PubMed.
- E.-S. Lee, H.-S. Yeom, J.-H. Hwang and S. Shin, Eur. J. Org. Chem., 2007, 2007, 3503–3507 CrossRef PubMed.
- F. N. Shirota, E. G. DeMaster and H. T. Nagasawa, J. Med. Chem., 1979, 22, 463–464 CrossRef CAS.
- P. H. Yu, B. A. Davis and A. A. Boulton, J. Med. Chem., 1992, 35, 3705–3713 CrossRef CAS.
- C. Wei and C.-J. Li, J. Am. Chem. Soc., 2002, 124, 5638–5639 CrossRef CAS PubMed.
- C. Fischer and E. M. Carreira, Org. Lett., 2001, 3, 4319–4321 CrossRef CAS PubMed.
- Y. Imada, M. Yuasa, I. Nakamura and S.-I. Murahashi, J. Org. Chem., 1994, 59, 2282–2284 CrossRef CAS.
- C. Wei and C.-J. Li, J. Am. Chem. Soc., 2003, 125, 9584–9585 CrossRef CAS PubMed.
- S. Sakaguchi, T. Kubo and Y. Ishii, Angew. Chem., Int. Ed., 2001, 113, 2602–2604 CrossRef.
- X. Zhang and A. Corma, Angew. Chem., Int. Ed. Engl., 2008, 47, 4358–4361 CrossRef CAS PubMed.
- K. Namitharan and K. Pitchumani, Eur. J. Org. Chem., 2010, 2010, 411–415 CrossRef PubMed.
- T. Zeng, W.-W. Chen, C. M. Cirtiu, A. Moores, G. Song and C.-J. Li, Green Chem., 2010, 12, 570–573 RSC.
- Y. Zhang, P. Li, M. Wang and L. Wang, J. Org. Chem., 2009, 74, 4364–4367 CrossRef CAS PubMed.
- B. Sreedhar, A. S. Kumar and P. S. Reddy, Tetrahedron Lett., 2010, 51, 1891–1895 CrossRef CAS PubMed.
- B. M. Choudary, C. Sridhar, M. L. Kantam and B. Sreedhar, Tetrahedron Lett., 2004, 45, 7319–7321 CrossRef CAS PubMed.
- B. Karimi, M. Gholinejad and M. Khorasani, Chem. Commun., 2012, 48, 8961–8963 RSC.
- N. Mizuno and M. Misono, Chem. Rev., 1998, 98, 199–218 CrossRef CAS PubMed.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS.
- M. Abdollahi-Alibeik and A. Rezaeipoor-Anari, Catal. Sci. Technol., 2014, 4, 1151–1159 CAS.
- M. Abdollahi-Alibeik and M. Pouriayevali, Catal. Commun., 2012, 22, 13–18 CrossRef CAS PubMed.
- B. Chakraborty and B. Viswanathan, Catal. Today, 1999, 49, 253–260 CrossRef CAS.
- J. Demel, J. Čejka and P. Štěpnička, J. Mol. Catal. A: Chem., 2007, 274, 127–132 CrossRef CAS PubMed.
- J. C. Juan, J. Zhang and M. A. Yarmo, J. Mol. Catal. A: Chem., 2007, 267, 265–271 CrossRef CAS PubMed.
- M. H. Al-Hazmi and A. W. Apblett, Catal. Sci. Technol., 2011, 1, 621–630 CAS.
- C.-W. Jiang, X. Zhong and Z.-H. Luo, RSC Adv., 2014, 4, 15216–15224 RSC.
- X. Dong, Y. Hui, S. Xie, P. Zhang, G. Zhou and Z. Xie, RSC Adv., 2013, 3, 3222–3226 RSC.
- F. Farzaneh, E. Zamanifar and C. D. Williams, J. Mol. Catal. A: Chem., 2004, 218, 203–209 CrossRef CAS PubMed.
- J.-S. Choi, S.-S. Yoon, S.-H. Jang and W.-S. Ahn, Catal. Today, 2006, 111, 280–287 CrossRef CAS PubMed.
- L. Wang, A. Kong, B. Chen, H. Ding, Y. Shan and M. He, J. Mol. Catal. A: Chem., 2005, 230, 143–150 CrossRef CAS PubMed.
- S. Vetrivel and A. Pandurangan, J. Mol. Catal. A: Chem., 2005, 227, 269–278 CrossRef CAS PubMed.
- V. Cortés Corberán, M. J. Jia, J. El-Haskouri, R. X. Valenzuela, D. Beltrán-Porter and P. Amorós, Catal. Today, 2004, 91–92, 127–130 CrossRef PubMed.
- Á. Szegedi, M. Popova, V. Mavrodinova, M. Urbán, I. Kiricsi and C. Minchev, Microporous Mesoporous Mater., 2007, 99, 149–158 CrossRef PubMed.
- S. Higashimoto, Y. Hu, R. Tsumura, K. Iino, M. Matsuoka, H. Yamashita, Y. G. Shul, M. Che and M. Anpo, J. Catal., 2005, 235, 272–278 CrossRef CAS PubMed.
- V. Ritleng, C. Sirlin and M. Pfeffer, Chem. Rev., 2002, 102, 1731–1770 CrossRef CAS PubMed.
- S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234–6458 CrossRef CAS PubMed.
- L. R. Pizzio, P. G. Vázquez, C. V. Cáceres and M. N. Blanco, Appl. Catal., A, 2003, 256, 125–139 CrossRef CAS.
- M. J. Albaladejo, F. Alonso, Y. Moglie and M. Yus, Eur. J. Org. Chem., 2012, 2012, 3093–3104 CrossRef CAS PubMed.
- M. J. Aliaga, D. J. Ramon and M. Yus, Org. Biomol. Chem., 2010, 8, 43–46 CAS.
- M. Lakshmi Kantam, S. Laha, J. Yadav and S. Bhargava, Tetrahedron Lett., 2008, 49, 3083–3086 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2014 |