Preparation of hierarchically structured anodic aluminum oxide by a hexagonal embedded nanosphere array†
Abstract
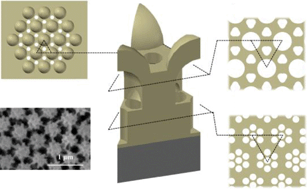
This study explored a built-in nanosphere template for anodic aluminum oxide (AAO) preparation and a hierarchically structured AAO with multilayered channels was achieved. A “defect anodization” mechanism based on a new voltage/interpore distance relationship was proposed and would be a reference for the AAO preparation based on pre-patterning methods.

 Please wait while we load your content...
Please wait while we load your content...