Lifetime-ultra-prolonged luminescent multilayer thin films with electronic microenvironment†
Tianlei Wang,
Meitang Liu*,
Hongwen Ma,
Xiaojuan Liu,
Yu Fu and
Kunran Hu
School of Materials Science and Technology, China University of Geosciences, Beijing 100083, P. R. China. E-mail: mtliu@cugb.edu.cn; Fax: +86-10-8232-2759; Tel: +86-10-8232-2759
First published on 13th August 2014
Abstract
In this report, luminescent multilayer thin films (MTFs) based on functional molecules intercalated into layered double hydroxides (LDHs) and montmorillonite (MMT) nanosheets were fabricated by layer-by-layer assembly method (LBL method). Exfoliated LDHs and MMT nanosheets with opposite charges can be expected to provide an electronic microenvironment (EME) for chromophores, which are not found in previous literatures, and to offer the inorganic rigid building blocks at the same time. Surprisingly, the lifetimes of MTFs with EME's architecture are significantly prolonged, compared with that of the pristine powder, even considerably longer than those of the MTFs without EME's architecture. Therefore, it is highly expected that the EME formed by oppositely-charged inorganic nanosheets has remarkable influence on enhancing the lifetimes of chromophores, which suggests a new potential method to develop novel light-emitting materials and optical devices.
1. Introduction
Organic luminescent materials, because of their chemical stability and excellent luminescence properties, give rise to many possible applications in chemical sensors,1,2 optical devices,3–7 photovoltaic cells8,9 etc. Compared with their solution counterparts, the luminescence performances of solid-state organic materials are still greatly limited by their poor optical stabilities and relatively short service lifetime.10 For example, the phenomena of fluorescence red or blue shift, broadening, or even quenching, can still occur in the solid state because of the formation of aggregates of such species.10–12 Accordingly, the group of Tolbert used the oriented mesoporous silica composites to control the flow of energy transfer, which provide insights to optimize nano-structured materials used in optoelectronic devices,13,14 and then other scientists tried changing the external factors, such as thermal and mechanical stimuli, to modify the chemical structure of the molecules for luminescent materials.15–18Nowadays, layered materials, such as layered double hydroxides,19,20 montmorillonite,21,22 perovskite oxides,23,24 and graphene25,26 are attractive targets for both fundamental research and practical application. As a successful liquid exfoliation of layered materials27–29 and a productive assembly of inorganic nanosheets,30–32 LBL method has been used to build layered and ordered functional films. Recently, several groups have performed many profound researches by using nanosheets with two-dimensional arrays, which can provide a rigid and stable environment.33–38 Especially, Duan et al. used LDHs nanosheets to suppress the chromophores' π–π stacking, and assembled well-oriented photoemissive structures with a macroscopic polarized optical effect.39–42 Various different inorganic nanosheets possibly contain a number of charges, such as MMT's negative charge and LDHs' positive charge. Oppositely-charged nanosheets are expected to form a nano-scaled capacitor providing an EME, which is not reported in any previous literatures (Fig. 1). Herein, our interest is in knowing whether functional molecules can be designed for intercalating into the oppositely-charged LDHs and MMT nanosheets, and how the EME affects the properties of functional molecules.
In this work, a photoactive divalent cation bis(N-methylacridinium)(BNMA) was intercalated into the LDHs nanosheets and MMT nanosheets, using an optically-inert polyvinyl alcohol (PVA) as bonders for the purpose of fabricating MTFs (Fig. 2). As the assembled nano-scaled capacitor can provide EME to the chromophores in the nano-system, the MTFs exhibit remarkable optical properties with reasonably longer luminescent lifetimes. Herein, this work successfully developed a general and facile method to fabricate novel inorganic–organic luminescent MTFs containing oppositely-charged nanosheets with a suitable and optically-inert polymer as the bonder and puts forward a new concept about the nano-scale capacitor's effects on the chromophores.
2. Results and discussion
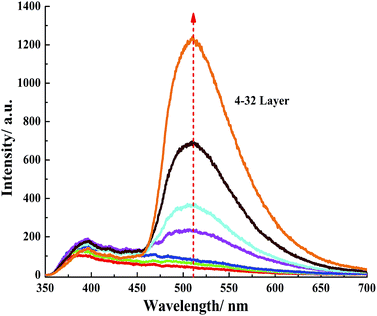
The UV-visible absorption spectra of (MMT/BNMA@PVA/LDHs/BNMA@PVA)n MTFs with varying numbers of assembly steps are shown in Fig. 3. The intensities of the absorption peaks at 432 nm exhibit linear correlation with the layer number n (Fig. 3, inset), which demonstrates a stepwise and regular growth procedure. Furthermore, the fluorescence emission intensity at 510 nm also displays a consistent increase with n, as shown in Fig. 4. The photoluminescence spectra of the as-prepared MTFs with different layer number have no obvious red or blue shift, which implies the absence of BNMA aggregates throughout the whole assembly process.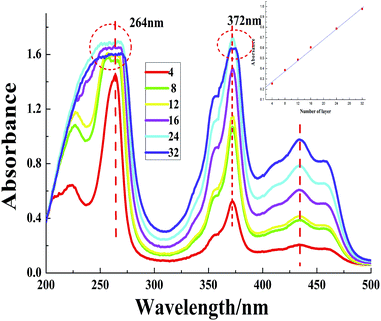 | ||
| Fig. 3 UV-visible absorption spectra of (MMT/BNMA@PVA/LDHs/BNMA@PVA)n MTFs. The inset shows the absorbance increasing linear relationship in 432 nm. | ||
To gain insight into the photophysical properties, the MTFs were studied by detecting the luminescence lifetimes. Surprisingly, it is interesting that the lifetimes of (MMT/BNMA@PVA/LDHs/BNMA@PVA)n MTFs were prolonged nearly 40-fold (13.81–17.02 ns) (Table 1, ESI Fig. S1†) compared with the pristine powder BNMA (0.37 ns), and were also prolonged 3-fold compared with the LDHs/BNMA@PVS MTFs (4.61–4.88 ns).40 This remarkable increase was partially because of the isolation effect (IE) caused by the rigid LDHs and MMT monolayer, preventing the aggregation of chromophores. Most importantly, positively charged LDHs and negatively charged MMT nanosheets formed a nano-scaled capacitor, thus providing BNMA with an EME that could apparently affect the vibration of the valence electron of the chromophores, which will definitely prolong their lifetimes. In order to verify this phenomenon, (MMT/BNMA@PVA)n MTFs were fabricated using LBL method, and their lifetimes are shown in ESI Table S2 and Fig. S2.† Combined with the results of these MTFs' lifetimes, it is obvious that the lifetimes of MTFs are nearly in a stable growth, but the (MMT/BNMA@PVA)n MTFs' lifetimes increase was linear and more steep. Furthermore, the (MMT/BNMA@PVA/LDHs/BNMA@PVA)n MTFs' lifetimes were obviously longer than (MMT/BNMA@PVA)n MTFs' in the same cycles. According to this remarkable increase, as mentioned in the above theory, it can be reasonably concluded that EME can definitely prolong chromophores' lifetimes. Fig. 5 illustrates the comparison of chromophores' lifetimes at different states under different environments, and it is obvious that the chromophores under EME have amazing ultra-prolonged lifetimes. Moreover, these results support that the EME has remarkably effective influence on enhancing the lifetime of the chromophores.
3. Experimental section
3.1 Reagents and materials
All the chemicals were of analytical grade and used as received without further purification. Bis(N-methylacridinium) (BNMA) was purchased from Sigma Chemical. Co. Ltd, and polyvinyl alcohol (PVA, MW = 1750) was purchased from Tianjin Fuchen Chemical Reagent Plant. Na-montmorillonite (MMT) was purchased from Zhejiang Sanding Co. Ltd. Mg(NO3)2·6H2O, Al(NO3)3·9H2O were all supplied by the Xilong Chemical Plant. NaOH, H2O2 (30%), H2SO4 (95–98%) were supplied by the Beijing Chemical Reagent Company.3.2 Characterization
UV-visible absorption spectra were obtained in the range from 200 to 500 nm on a TU-1901 Double beam UV-vis spectrophotometer with a slit width of 2.0 nm. Fluorescence spectra was recorded on F-4600 Fluorospectrophotometer. The fluorescence emission spectra were in the range from 350 to 700 nm, and both the excitation and emission slit were set to 2.0 nm. The fluorescence decay and polarized photoluminescence measurements of MTFs were recorded by using an Edinburgh Instruments' Steady and transient time-resolved fluorescence spectrometer.3.3 Fabrication of (MMT/BNMA@PVA/LDHs/BNMA@PVA)n MTFs
A mixed aqueous nitrate solution (Mg/Al molar ratio equals 2.0) was prepared, and the concentration of Mg2+ was 0.8 mol L−1. The solution of sodium hydroxide (2.4 mol L−1) was mixed with the salt solution at the same rate with vigorous stirring. Then the suspension was stirred at 80 °C for 24 h under nitrogen gas flow. The precipitate was centrifuged, washed with hot distilled water and dried under vacuum at 60 °C.The product (0.1 g) LDHs was mixed with 100 mL formamide in a flask, which was tightly sealed after purging with nitrogen gas. The mixture was vigorously shaken by a mechanical shaker at a speed of 160 rpm for 2 days. The upper suspension with exfoliated LDHs nanosheets were obtained after centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The MMT powder (1 g) was dissolved in 1000 mL distilled water. After vigorous stirring for 4 weeks, the insoluble fraction was allowed to deposit for 24 h and the suspension with exfoliated MMT nanosheets was collected for subsequent use. Quartz slides (1.5 × 1.5 cm2) were cleaned in a “piranha” solution (H2SO4
000 rpm for 10 min. The MMT powder (1 g) was dissolved in 1000 mL distilled water. After vigorous stirring for 4 weeks, the insoluble fraction was allowed to deposit for 24 h and the suspension with exfoliated MMT nanosheets was collected for subsequent use. Quartz slides (1.5 × 1.5 cm2) were cleaned in a “piranha” solution (H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 = 3
H2O2 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume), and then thoroughly rinsed with distilled water and dried under nitrogen flow. PVA was dissolved in distilled water to obtain 1 wt% aqueous solution, the BNMA (1 g) was dissolved in 100 mL of distilled water, then the solution of BNMA and PVA were mixed (1
1 in volume), and then thoroughly rinsed with distilled water and dried under nitrogen flow. PVA was dissolved in distilled water to obtain 1 wt% aqueous solution, the BNMA (1 g) was dissolved in 100 mL of distilled water, then the solution of BNMA and PVA were mixed (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) to form BNMA@PVA solution, which had 0.5 g L−1 of BNMA. The heterogeneous thin films were fabricated by applying a cyclic repetition of the following steps: (a) immersed the quartz slide into exfoliated MMT solution for 5 min, then thoroughly rinsed it with distilled water and dried it at room temperature; (b) dipped it into an aqueous solution of BNMA@PVA for 5 min, then thoroughly rinsed it with distilled water and dried it at room temperature; (c) dipped it into exfoliated LDHs suspension for 5 min, then rinsed it with distilled water and dried it as mentioned above; (d) dipped it into an aqueous solution of BNMA@PVA for 5 min, then washed with distilled water and dried it. All these procedures were repeated as necessary to obtain the desired number of (MMT/BNMA@PVA/LDHs/BNMA@PVA)n.
1 in volume) to form BNMA@PVA solution, which had 0.5 g L−1 of BNMA. The heterogeneous thin films were fabricated by applying a cyclic repetition of the following steps: (a) immersed the quartz slide into exfoliated MMT solution for 5 min, then thoroughly rinsed it with distilled water and dried it at room temperature; (b) dipped it into an aqueous solution of BNMA@PVA for 5 min, then thoroughly rinsed it with distilled water and dried it at room temperature; (c) dipped it into exfoliated LDHs suspension for 5 min, then rinsed it with distilled water and dried it as mentioned above; (d) dipped it into an aqueous solution of BNMA@PVA for 5 min, then washed with distilled water and dried it. All these procedures were repeated as necessary to obtain the desired number of (MMT/BNMA@PVA/LDHs/BNMA@PVA)n.
3.4 Fabrication of (MMT/BNMA@PVA)n MTFs
The processes of fabrication of (MMT/BNMA@PVA)n is similar to (MMT/BNMA@PVA/LDHs/BNMA@PVA)n, except for the aqueous nanosheets solution of LDHs.4. Conclusions
In brief, this work proposes a new method to assemble a novel luminescent MTFs with ultra-prolonged lifetime, as the chromophores were confined in the oppositely-charged inorganic layer hosts. It successfully demonstrates that the EME is sufficiently beneficial to enhancing the lifetimes of MTFs. Therefore, the as-fabricated MTFs are expected to have considerable flexibility and be potential for designing, constructing and investigating novel optoelectrical devices. Further work is going on with the research on lifetime of various luminescent guests imbedded in a series of different LDHs/MMT nanosheets with EME.Acknowledgements
This work was supported partially by the National Natural Science Foundation of China (Grant no. 40802013), Key Projects in the National Science & Technology Pillar Program (Grant no. 2006BAD10B04), the Fundamental Research Funds for the Central Universities (Grant no. 2011YXL058).Notes and references
- W. Samuel Thomas III, D. J. Guy and M. S. Timothy, Chem. Rev., 2007, 107, 1339 CrossRef PubMed.
- L. Jukka, S. Mikko, V. Antti, A. Timo, P. Janika, K. Natalia and K. Jouko, J. Am. Chem. Soc., 2001, 123, 6083 CrossRef PubMed.
- C. A. Strassert, C. H. Chien, M. D. G. Lopez, D. Kourkoulos, D. Hertel, K. Meerholz and D. C. Luisa, Angew. Chem., Int. Ed., 2011, 50, 946 CrossRef CAS PubMed.
- Q. S. Zhang, T. Komino, S. P. Huang, S. Matsunami, K. Goushi and C. Adachi, Adv. Funct. Mater., 2012, 22, 2327 CrossRef CAS PubMed.
- M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houk, Chem. Soc. Rev., 2009, 38, 1330 RSC.
- J. Schmidtke, W. Stille, H. Finkelmann and S. T. Kim, Adv. Mater., 2002, 14, 746 CrossRef CAS.
- Q. L. Niu, Y. Zhou, L. Wang, J. B. Peng, J. Wang, J. Pei and Y. Cao, Adv. Mater., 2008, 20, 964 CrossRef CAS PubMed.
- J. Piris, N. Kopidakis, D. C. Olson, S. E. Shaheen, D. S. Ginley and G. Rumbles, Adv. Funct. Mater., 2007, 17, 3849 CrossRef CAS PubMed.
- K. M. Jeremiah, R. P. Mauricio, W. David, A. Nisha, S. S. Kirk and R. R. John, Langmuir, 2005, 21, 10123 Search PubMed.
- A. L. Igor, K. Jinsang and M. S. Timothy, J. Am. Chem. Soc., 1999, 121, 1466 CrossRef.
- S. R. Amrutha and M. Jayakannan, J. Phys. Chem. B, 2008, 112, 1119 CrossRef CAS PubMed.
- C. Xia and R. C. Advincula, Macromolecules, 2001, 34, 5854 CrossRef CAS.
- T. Q. Nguyen, J. J. Wu, V. Doan, B. J. Schwartz and S. H. Tolbert, Science, 2000, 288, 652 CrossRef CAS.
- A. P. Clark, K. F. Shen, Y. F. Rubin and S. H. Tolbert, Nano Lett., 2005, 5, 1647 CrossRef CAS PubMed.
- M. Toshiki, S. Hiroyuki and A. Koji, Nat. Mater., 2005, 4, 685 CrossRef PubMed.
- Y. Sagara and T. Kato, Nat. Chem., 2009, 1, 605 CrossRef CAS PubMed.
- X. L. Luo, J. N. Li, C. H. Li, L. Q. Heng, Y. Q. Dong, Z. P. Liu, Z. S. Bo and B. Z. Tang, Adv. Mater., 2011, 23, 3261 CrossRef CAS PubMed.
- T. Mutai, H. Satou and K. Araki, Nat. Mater., 2005, 4, 685 CrossRef CAS PubMed.
- P. J. Sideris, U. G. Nielsen, Z. H. Gan and C. P. Grey, Science, 2008, 321, 113 CrossRef CAS PubMed.
- D. H. Park, S. J. Hwang, J. M. Oh, J. H. Yang and J. H. Choy, Prog. Polym. Sci., 2013, 38, 1442 CrossRef CAS PubMed.
- M. Ikeda, T. Yoshii, T. Matsui, T. Tanida, H. Komatsu and I. Hamachi, J. Am. Chem. Soc., 2011, 133, 1670 CrossRef CAS PubMed.
- M. T. Liu, M. F. Pu, H. W. Ma, Y. F. Hu, X. J. Liu and X. Pang, Polym. Compos., 2011, 32, 1002 CrossRef CAS PubMed.
- Y. Tokunaga, N. Furukawa, H. Sakai, Y. Taguchi, T. H. Arima and Y. Tokura, Nat. Mater., 2009, 8, 558 CrossRef CAS PubMed.
- A. Bera, H. Y. Peng, J. Lourembam, Y. D. Shen, X. W. Sun and T. Wu, Adv. Funct. Mater., 2013, 23, 4977 CrossRef CAS PubMed.
- L. Wang, I. Meric, P. Y. Huang, Q. Gao, Y. Gao, H. Tran, T. Taniguchi, K. Watanabe, L. M. Campos, D. A. Muller, J. Guo, P. Kim, J. Hone, K. L. Shepard and C. R. Dean, Science, 2013, 342, 6158 Search PubMed.
- X. Y. Dong, L. Wang, D. Wang, C. Li and J. Jin, Langmuir, 2012, 28, 293 CrossRef CAS PubMed.
- V. Nicolosi, M. Chhowalla, M. G. Kanatzidis, M. S. Strano and J. N. Coleman, Science, 2013, 340, 1420 CrossRef CAS.
- Z. P. Liu, R. Z. Ma, M. Osada, N. Iyi, Y. Ebina, K. K. Takada and T. Sasaki, J. Am. Chem. Soc., 2006, 128, 4872 CrossRef CAS PubMed.
- L. Li, R. Z. Ma, Y. Ebina, N. Iyi and T. Sasaki, Chem. Mater., 2005, 17, 4386 CrossRef CAS.
- P. Podsiadlo, A. K. Kaushik, E. M. Arruda, A. M. Waas, B. S. Shim, J. D. Xu, H. Nandivada, B. G. Pumplin, J. Lahann, A. Ramamoorthy and N. A. Kotov, Science, 2007, 318, 80 CrossRef CAS PubMed.
- V. Nicolosi, M. Chhowalla, M. G. Kanatzidis, M. S. Strano and J. N. Coleman, Science, 2013, 340, 1420 CrossRef CAS.
- S. Huang, X. Cen, H. D. Peng, S. Z. Guo, W. Z. Wang and T. X. Liu, J. Phys. Chem. B, 2009, 113, 15225 CrossRef CAS PubMed.
- D. M. Xu, M. Y. Guan, Q. H. Xu and Y. Guo, J. Hazard. Mater., 2013, 262, 64 CrossRef CAS PubMed.
- H. J. Li, G. Zhu, Z. H. Liu, Z. P. Yang and Z. L. Wang, Carbon, 2010, 48, 4391 CrossRef CAS PubMed.
- Q. Wang and D. O'Hare, Chem. Rev., 2011, 112, 4124 CrossRef PubMed.
- D. P. Yan, J. Lu, J. Ma, M. Wei, D. G. Evans and X. Duan, Angew. Chem., Int. Ed., 2011, 50, 720 CrossRef CAS PubMed.
- F. Leroux and C. Taviot-Gueho, J. Mater. Chem., 2005, 36258 Search PubMed.
- J. L. Gunjakar, T. W. Kim, H. N. Kim, I. Y. Kim and S. J. Hwang, J. Am. Chem. Soc., 2011, 133, 14998 CrossRef CAS PubMed.
- D. P. Yan, J. Lu, J. Ma, S. H. Qin, M. Wei, D. G. Evans and X. Duan, Angew. Chem., Int. Ed., 2011, 50, 7037 CrossRef CAS PubMed.
- D. P. Yan, J. Lu, L. Chen, S. H. Qin, J. Ma, M. Wei, D. G. Evans and X. Duan, Chem. Commun., 2010, 5912 RSC.
- D. P. Yan, J. Lu, M. Wei, J. B. Han, J. Ma, F. Li, D. G. Evans and X. Duan, Angew. Chem., Int. Ed., 2009, 48, 3073 CrossRef CAS PubMed.
- D. P. Yan, J. Lu, J. Ma, M. Wei, X. R. Wang, D. G. Evans and X. Duan, Langmuir, 2010, 26, 7007 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fluorescence lifetimes, parameters of multi-exponential fit to the fluorescence decay, and polarized fluorescence spectra of (MMT/BNMA@PVA/LDHs/BNMA@PVA)n and (MMT/BNMA@PVA)n MTFs. See DOI: 10.1039/c4ra05884d |
| This journal is © The Royal Society of Chemistry 2014 |