Self-assembled triphenylamine derivative for trace detection of picric acid†
Abstract
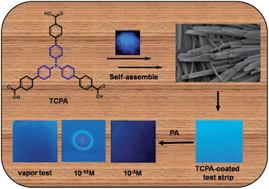
Detection of explosives is of utmost importance due to the threat to human security as a result of illegal transport and terrorist activities. Picric acid (PA) is widely used in the industrial and military fields, and is inadvertently able to contaminate the environment and groundwater, posing a threat to human health. Achieving the detection of explosives at the parts per billion (ppb) level using chemosensors is a great challenge. Herein, we demonstrate that triphenylamine based fluorescent chemosensors tris(4′-carbethoxybiphenyl) amine (TCEPA) and tris(4′-carboxybiphenyl) amine (TCPA) exhibit superior detection capability for PA in solution state at the ppb level (40 ppb and 5 ppb respectively) and efficient quenching behaviours in the vapor phase at room temperature. In addition, fluorescent test strips have been prepared by dip-coating Thin Layer Chromatography (TCL) for trace detection of PA at the attogram level.

 Please wait while we load your content...
Please wait while we load your content...