Surface passivation assisted lasing emission in the quantum dots doped cholesteric liquid crystal resonating cavity with polymer template†
Lin-Jer Chen*,
Chia-Rong Lee and
Chung-Liang Chu
Department of Photonics, National Cheng Kung University, Tainan, Taiwan 701, Taiwan. E-mail: crlee@mail.ncku.edu.tw
First published on 3rd October 2014
Abstract
A copolymer has been used as a template for enhanced optical properties of core–shell CdS/ZnSe quantum dots doped cholesteric liquid crystal. The lasing behavior is achieved at relatively low laser excitation energy threshold at room temperature. Low-threshold value and high-optical stability make this optical system promising for visible laser emitter applications.
Cholesteric liquid crystals (CLCs) are materials with a periodic dielectric structure, which leads to the formation of a photonic bandgap (PBG).1 CLCs phase inherently exhibits a selective reflection that is related to the Bragg optics associated with the helical structure formed from a one dimensional rotation of the birefringence axis.2 They have attracted much interest for their promising applications in many optical fields (such as lasing emission).3–6 Quantum dots (QDs) are semiconductor nanocrystals whose electrons and holes are quantum-confined in all three spatial dimensions. They have attracted much attention during the past few decades due to their unique optical properties as distinguished from the corresponding bulk materials and have shown great potential in the fabrication of next generation optoelectronic devices.7,8 In recent years, highly efficient quantum emitters have proven to be very attractive light sources.9 Special optical emission features from the QDs as an active medium are naturally expected due to their unique quantum structure that is different from the conventional organic dye molecules.10 The optical characteristics of a quantum dot doped CLCs microresonator inevitably change with varying operation template, which greatly determines its applications in fields requiring specific and stable performance. However, only a few studies have been reported on the soft matter dependence of optical characteristics of QDCLCs devices.10–12 In contrast with conventional organic dyes or fluorescent proteins, QDs possess unique optical properties. They have a broad absorption band, a narrow emission band, size-tunable emission from visible to near infrared range, superior brightness, long fluorescent lifetime, and more importantly, they are highly resistant to photobleaching and have relatively large surface area for functionalization.13 Another problem is that QDs tend to aggregate inside liquid crystals and are often trapped or sequestered in organelles. Owing to these problems, cationic dendrimers and micelles are unstable (e.g., aggregation) in soft matter or culture media and have decreasing emission performance.14,15 Such surface passivation has been achieved using organic capping agents as well as the formation of inorganic QDs systems such as CdSe/ZnSe.16–19 The 1-hexanethiol (C6H14S) surfactant also improves the dispersion and stability of the coated QDs in this case. Recently, many researchers have successfully combined liquid crystal materials with polymer template technology to fabricate optical devices using template agents as structure directing agents.20,21 Recently, polymer template assisted synthesis has been proven as a promising method to obtain soft materials with defined chemical and optical properties.22,23
In this work, we describe the template design and procedure to control the lasing emission of QDCLCs. This report includes guidelines to comprehend the correlation between the controlled QDCLCs stability and the lasing characteristics by band edge effect, providing an available route to fabricate novel active laser devices in template system. At the same time, the stable pumped energy of 11 μJ per pulse is obtained, which is 2 times better than that of reference QDCLCs band-edge laser without template. The broad gain profile and template polymerization effect in QDCLCs laser are likely playing an important role in reduction of the quantum dots' aggregate sensitivity of the lasing efficiency.
Fig. 1(a) and S2† show photoluminescence (PL) and UV absorption spectra of CdSe/ZnSe QDs (before surface passivation is black line, after surface passivation is red line) dispersed in toluene. As shown, both the PL and UV spectra from the core–shell QDs essentially maintain their overall shape with absorption decay and enhanced intensity compared to those of QDs before surface passivation. The optical properties changed are an indication of the aggregation of core–shell QDs, which has been described in detail by several authors.24–26 Although the experimental conditions have not been optimized, the PL peak intensity increased more than 3 times after surface passivation with a core–shell of QDs, compared to that of QDs before surface passivation, without significant modifications of the absorbance and PL features. In addition, the position of the PL peak remained essentially unchanged during the surface passivation process, indicating that the QDs retained substantial optical properties. We concluded that the spectral blue shifts of QDs were not due to the presence of QDs aggregates and gradual dissociation of aggregates.27 This was confirmed from the PL and absorption spectra of QD solutions with surface passivation: the PL intensities of QD solutions decreased without surface passivation. From these observations, we concluded not only that the aggregation of the QDs was minimized under the experimental conditions but also that the increase of the PL intensity was due to the presence of aggregates or changes in the physical properties such as pitch and dipole moment of the medium. However, we observed considerable decreases of the PL emission intensity of the QDs when photoactivated without surface passivation; the PL efficiency decreased about 23.1% in toluene and 61.5% in CLCs polymer template (Fig. 1(b)). One series of QDCLCs fluorescence spectra after passivation of QDs doping and polymer template formed is shown in Fig. 1(b), where text in each step (b1–b5) indicates the PL intensity of QDCLCs after each condition. It was mentioned that PL intensity of QDCLCs increases remarkably with the passivation of the QDs in the CLCs template. It could be affected by structural changes during the polymer template growth and/or doping by passivation of QDs. The result clearly demonstrates that passivation and template treated enhance the QD fluorescence, but they do not affect the wavelength of the QD fluorescence peak. Most importantly, such modification is notable; i.e., the PL intensity is totally recovered after polymer template is added to passivation of QDs in the CLC system, which could be luminescence centers as well. On the other hand, the lasing emission can only be obtained when the QDs were used by surface passivation in the template mode. The decreases of the PL or lasing efficiency in this case can be attributed to poor photoactivated surface passivation and rapid formation of aggregation in the template sample. Also, the increase of the optical efficiency is due to a decrease in the number of surface defects or an increase in the density of emissive surface states. It should be noted that, after the surface passivation process, the lasing intensity of the sample increased slightly with QDs emission matching the band edge of the CLCs polymer template. Therefore, the contribution of a template component to the interactions between CLCs and the surface of the QDs cannot be completely ruled out.
Fig. S3† indicates the reflection spectra of the sample measured at the before-curing, after-curing, after-washing, and after-refilling stages of fabrication of QDNLC (nematic liquid crystal)-refilling template sample, presented by black, green, red, and blue curves, respectively. Apparently, the spectral positions of the PBG of the sample and thus its central wavelength (λc) are distinct at different stages. The λc of the PBG of the sample shifts slightly from 640 nm to 590 nm after UV-curing. This result is attributable to the slight decrease of helical pitch of the chiral polymer following the volume shrinkage through the polymerization and crosslinking reactions.20 Fig. S4(a)–(d)† shows the optical properties and morphologies in QDCLCs devices as observed using a polarizing optical microscope (POM). It is to be noted that the almost appearance blue shift of the reflect band in the template mode is a sensitive response of the polymerization of the QDCLCs .28
Fig. 2 shows optical and fluorescent microscope images of QDCLCs polymer template. Before growth of the QDCLCs polymer template, the QDCLCs device showed PL emission but with quite low PL intensity due to more aggregation of QDs as shown in Fig. 2(a). After QDCLCs polymer template growth (less aggregation of QDs, Fig. 2(b)), the lasing properties of the resulting polymer template became quite good. Without substantial optimization, the PL efficiency increased more than 1.3 times and the lasing emission appears. As mentioned above, we believe the occurrence of aggregation originated from interparticle hydrogen bonding. The results also suggested the disaggregation was not a result of the repulsion of an electrostatic force between interparticles. The QDs of the CLC polymer template are well separated from each other, proving that QDs have been well protected by surface passivation and successfully embedded into the polymer template. From these observations, we concluded not only that the aggregation of the QDs was minimized under the polymer template conditions but also that the increase in the PL intensity was mainly due to the dissociation of aggregates or changes in the physical properties such as dipole moment of the medium. The decreases of the PL intensity in this case can be attributed to poor surface passivation and formation of the surface defects in these soft matters. In addition, the stop band of the QDCLC polymer template formed with increasing static time. Over a broad range of static time (3 h), we observed a complete stop band or no change in the characteristics of the band-edge for QDCLC polymer template (Fig. S5†). The above results indicate that static time is the crucial factor for this system.
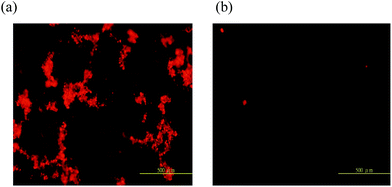 | ||
| Fig. 2 Fluorescent microscopy of QDCLCs polymer template devices (a) without polymer template and (b) with polymer template. | ||
Fig. 3(a) shows the lasing intensity spectrum of QDCLCs polymer template structure and corresponding reflection spectra (dashed line). The raise in intensity due to selective reflection was observed around 608 nm, which corresponds to the longer wavelength edge of the CLCs. The decrease in transmittance at shorter wavelength is attributed to the absorption of the doped QDs. It should be noted that the lasing peak wavelength at 608 nm matches with the band edge of the CLCs. From this result, it is found that this laser action is based on the contribution of the photonic band edge of the CLCs cell. The polarization analysis of QDCLCs polymer template lasing has been investigated for left- and right-circular polarizers (Fig. S6†). Fig. 3(b) shows the dependence of emission intensity on the pumping energy of Nd:YAG pulse laser and it is the spectral linewidth of the QDCLCs polymer template. This indicates the clear presence of a pumping threshold of approximately 11 μJ per pulse for laser action. The pumping energy dependence of emission intensity and the peak width (the full width at half maximum; FWHM) of the emission peak at 608 nm. At a low pumping energy, emission intensity increases in proportion to pumping energy. Above a threshold at the pumping energy of 11 μJ per pulse, emission intensity nonlinearly increases and a sharp emission peak appears. The FWHM of the peak is less than 2.0 nm above the threshold excitation energy. This behavior is similar to the line narrowing expected for amplified spontaneous emission (ASE). From the results, it was confirmed that the band edge effect of the QDCLC polymer template was effective on the laser action in the semiconductor compound structure with surface passivation. Fig. S7† indicates that the high-energy pulses that induced the thermal effect for dyes must be very strong to instantaneously evaporate the CLC material. The DDCLC laser on the pumped region therefore becomes totally destructive at the damage threshold of 55 μJ per pulse. By contrast, the QDCLC laser was not destroyed and could be repetitively used in the pumped region even after the excitation of the high-energy pulses on the cell at E = 100 μJ per pulse. ASE emerged at the reflect band edge of photonic due to the enhanced density of the optical state near the stop band edges.29 The experimental steady-state diffraction pattern of the QDCLCs polymer template is presented in Fig. S8,† and the respective lasing patterns of the samples are shown in the insets. As a result, the optical properties of the QDs as a gain medium effectively dissipate within the polymer template matrix without surface passivation. In fact, adding QDs with surface passivation into the polymer template was demonstrated as a more effective way to reduce photoemission quenching than organic dye.30 Another study reported that the PL intensity of QDs in colloidal solution can be significantly enhanced by adding proper polymers.31
Conclusions
We have successfully demonstrated the lasing emission in the QDCLCs polymer template. The device was fabricated by surface passivation QDs doped CLC with a polymer template. Lasing emission emerged at the reflect band edges of the QDCLCs polymer template, and the threshold was much lower when template assisted than without template, which was attributed to the increase of the local stability and density of state at the reflect band edges. The lasing threshold was 11 μJ per pulse, and the FWHM was 2 nm. Our experimental results could open up a broad search for developing high efficiency QDCLCs lasers.Acknowledgements
The authors would like to thank the Ministry of Science and Technology, Taiwan (MOST)(contract MOST 103-2112-M-006-012-MY3 and 103-2112-M-006-016-MY3) and the Advanced Optoelectronic Technology Center, National Cheng Kung University, under projects from the Ministry of Education, for financially supporting this research.Notes and references
- L. J. Chen, J. D. Lin, S. Y. Huang, T. S. Mo and C. R. Lee, Adv. Opt. Mater., 2013, 1, 637–643 CrossRef.
- H. Finkelmann, S. T. Kim and A. Munoz, Adv. Mater., 2001, 13, 1069 CrossRef CAS.
- P. V. Shibaev, J. Madsen and A. Z. Genack, Chem. Mater., 2004, 16, 1397–1399 CrossRef CAS.
- P. V. Shibaev, V. I. Kopp and A. Z. Genack, J. Phys. Chem. B, 2003, 107, 6961–6964 CrossRef CAS.
- Y. Wang, T. Manabe, Y. Takanishi, K. Ishikawa, G. Shao, A. Orita, J. Otera and H. Takezoe, Opt. Commun., 2007, 280, 408–411 CrossRef CAS PubMed.
- L. J. Chen, J. D. Lin and C. R. Lee, J. Mater. Chem. C, 2014, 2, 4388–4394 RSC.
- V. I. Klimov, S. A. Ivanov, J. Nanda, M. Achermann, I. Bezel, J. A. McGuire and A. Piryatinski, Nature, 2007, 447(7143), 441–446 CrossRef CAS PubMed.
- M. C. Schlamp, X. G. Peng and A. P. Alivisatos, J. Appl. Phys., 1997, 82(11), 5837–5842 CrossRef CAS PubMed.
- S. Reitzenstein, T. Heindel, C. Kistner, A. Rahimi-Iman, C. Schneider, S. Höfling and A. Forchel, Appl. Phys. Lett., 2008, 93, 061104 CrossRef PubMed.
- A. L. Rodarte, C. Gray, L. S. Hirst and S. Ghosh, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 035430 CrossRef.
- S. G. Lukishova, L. J. Bissell, J. Winkler and C. R. Stroud Jr, Opt. Lett., 2012, 37, 1259 CrossRef PubMed.
- A. Bobrovsky, K. Mochalov, V. Oleinikov, A. Sukhanova, A. Prudnikau, M. Artemyev, V. Shibaev and I. Nabiev, Adv. Mater., 2012, 24, 6216 CrossRef CAS PubMed.
- I. L. Medintz, H. T. Uyeda, E. R. Goldman and H. Mattoussi, Nat. Mater., 2005, 4(6), 435–446 CrossRef CAS PubMed.
- A. M. Smith, H. W. Duan, M. N. Rhyner, G. Ruan and S. M. Nie, Phys. Chem. Chem. Phys., 2006, 8, 3895 RSC.
- A. K. Patri, J. F. Kukowska-Latallo and J. R. Baker Jr, Adv. Drug Delivery Rev., 2005, 57, 2203 CrossRef CAS PubMed.
- D. V. Talapin, A. L. Rogach, A. Kornowski, M. Haase and H. Weller, Nano Lett., 2001, 1, 207 CrossRef CAS.
- X. Peng, M. C. Schlamp, A. V. Kadavanich and A. P. Alivisatos, J. Am. Chem. Soc., 1997, 119, 7019 CrossRef CAS.
- M. A. Hines and P. Guyot-Sionnest, J. Phys. Chem., 1996, 100, 468 CrossRef CAS.
- M. Danek, K. F. Jensen, C. B. Murray and M. G. Bawendi, Chem. Mater., 1996, 8, 173 CrossRef CAS.
- M. Mitov and N. Dessaud, Nat. Mater., 2006, 5, 361–364 CrossRef CAS PubMed.
- I. Dierking, L. L. Kosbar, A. Afzali-Ardakani, A. C. Lowe and G. A. Held, Appl. Phys. Lett., 1997, 71, 2454–2457 CrossRef CAS PubMed.
- S. S. Choi, S. M. Morris, W. T. S. Huck and H. J. Coles, Adv. Mater., 2010, 22, 53–56 CrossRef CAS PubMed.
- J. Guo, H. Cao, J. Wei, D. Zhang, F. Liu, G. Pan, D. Zhao, W. He and H. Yang, Appl. Phys. Lett., 2008, 93, 201901 CrossRef PubMed.
- V. Biju, R. Kanemoto, Y. Matsumoto, S. Ishii, S. Nakanishi, T. Itoh, Y. Baba and M. Ishikawa, J. Phys. Chem. C, 2007, 111, 7924–7932 CAS.
- S. Kim and M. G. Bawendi, J. Am. Chem. Soc., 2003, 125, 14652–14653 CrossRef CAS PubMed.
- Z. A. Peng and X. G. Peng, J. Am. Chem. Soc., 2001, 123, 183–184 CrossRef CAS.
- A. M. Derfus, W. C. W. Chan and S. N. Bhatia, Nano Lett., 2004, 4(1), 11–18 CrossRef CAS.
- M. Mitov and N. Dessaud, Liq. Cryst., 2007, 34, 183 CrossRef CAS.
- R. V. Nair, A. K. Tiwari, S. Mujumdar and B. N. Jagatap, Phys. Rev. A, 2012, 85, 023844 CrossRef.
- S. Singamaneni, C. Jiang, E. Merrick, D. Kommireddy and V. V. Tsukruk, J. Macromol. Sci., Part B: Phys., 2007, 46, 7 CrossRef CAS.
- A. A. Bol and A. Meijerink, J. Phys. Chem. B, 2001, 105, 10203 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental setup, PL spectra, polarizing microscopy image, lasing spectra, experimental section. See DOI: 10.1039/c4ra05787b |
| This journal is © The Royal Society of Chemistry 2014 |


