Chemistry, nanostructure and magnetic properties of Co–Ru–B–O nanoalloys†
G. M. Arzaca,
T. C. Rojas*a,
L. C. Gontarda,
L. E. Chinchillab,
E. Otalcd,
P. Crespoef and
A. Fernándeza
aInstituto de Ciencia de Materiales de Sevilla (CSIC-Univ. Sevilla), Avda. Américo Vespucio 49, 41092-Sevilla, Spain. E-mail: tcrojas@icmse.csic.es
bDepartamento de Ciencia de Materiales, Ingeniería Metalúrgica y Química Inorgánica, Facultad de Ciencias, Universidad de Cádiz, 11510 Puerto Real, Cádiz, Spain
cDivision of Porous Materials, UNIDEF, CITEDEF, CONICET, S. J. B de la Salle 4397, Villa Martelli (B1603ALO), Buenos Aires, Argentina
dLaboratory for Materials Science and Technology, FRSC-UTN, Av. Inmigrantes 555, Río Gallegos (9400), Argentina
eInstituto de Magnetismo Aplicado (RENFE-UCM-CSIC), P.O. Box 155, 28230 Las Rozas, Madrid, Spain
fDepartamento de Física de Materiales, Universidad Complutense, Madrid, Spain
First published on 9th September 2014
Abstract
In our previous works, Co–B–O and Co–Ru–B–O ultrafine powders with variable Ru content (xRu) were studied as catalysts for hydrogen generation through sodium borohydride hydrolysis. These materials have shown a complex nanostructure in which small Co–Ru metallic nanoparticles are embedded in an amorphous matrix formed by Co–Ru–B–O based phases and B2O3. Catalytic activity was correlated to nanostructure, surface and bulk composition. However, some questions related to these materials remain unanswered and are studied in this work. Aspects such as: 3D morphology, metal nanoparticle size, chemical and electronic information on the nanoscale (composition and oxidation states), and the study of the formation or not of a CoxRu1−x alloy or solid solution are investigated and discussed using XAS (X-ray Absorption Spectroscopy) and Scanning Transmission Electron Microscopy (STEM) techniques. Also magnetic behavior of the series is studied for the first time and the structure–performance relationships discussed. All Co-containing samples exhibited ferromagnetic behavior up to room temperature while the Ru–B–O sample is diamagnetic. For the xRu = 0.13 sample, an enhancement in the Hc (coercitive field) and Ms (saturation magnetization) is produced with respect to the monometallic Co–B–O material. However this effect is not observed for samples with higher Ru content. The presence of the CoxB-rich (cobalt boride) amorphous ferromagnetic matrix, very small metal nanoparticles (Co and CoxRu(1−x)) embedded in the matrix, and the antiferromagnetic CoO phase (for the higher Ru content sample, xRu = 0.7), explain the magnetic behavior of the series.
1. Introduction
The depletion of fossil fuels together with the environmental impact related to the emission of carbon dioxide and other contaminants makes necessary the research on new energy sources.1 In this context, H2 appears as a clean energy carrier with a high energy density (142 MJ kg−1 while for liquid hydrocarbons it is 47 MJ kg−1).2 The development of a hydrogen economy faces challenges related to H2 production (preferentially from renewable resources), transport and storage. For chemical hydrogen storage, great efforts have been made in these years to develop suitable materials with high gravimetric and volumetric density. In this direction, boron based hydrides are very attractive because of the combination of the lightweight of B and their high hydrogen content.3 Sodium borohydride (SB) is one of the most studied because releases hydrogen safely through its hydrolysis reaction (1) with a high potential hydrogen storage capacity (10.8%, but usually lower in experimental conditions due to the formation and precipitation of hydrated borates).3–7 Also methanolysis (reaction of SB with methanol) and the use of methanol–water mixtures were proposed as efficient methods to produce hydrogen from sodium borohydride with promising hydrogen storage capacity.8| NaBH4 + 2H2O → 4H2 + NaBO2 | (1) |
Uncatalysed, reaction (1) is very slow. For this reason, many acid and metal catalysts have been tested and/or prepared in these years.4–6 Most proposed systems for hydrogen generation through (1) are based on the addition of stabilized (on sodium hydroxide) SB solutions to a certain catalyst or by addition of water to a mixture of solid SB and catalyst.4–6,8 Cobalt is definitely the most studied metal catalyst because of its cost effectiveness.9–11 Co based catalysts have been tested and/or prepared in a wide of range of forms, from the simplest CoCl2·6H2O salt to metallic cobalt, cobalt oxides and also alloyed with other elements.12–17 Co–B based nanoalloys are the most employed in literature.9–11 Reaction of a cobalt precursor with sodium borohydride in aqueous medium leads to the formation of ultrafine and usually amorphous powders (Co–B materials) with enhanced activity not only for reaction (1) but also for many organic reactions.9–11,18,19 Despite being prepared and used in a good number of papers, the exact nature of these nanoalloys is still under intense discussion.9–11,18 Their amorphous and/or nanocrystalline character, their compositional complexity and the wide range of reaction conditions reported in literature, make difficult the study and comparison of reported results. Recently, we studied a Co–B–O and a series of Co–Ru–B–O materials as catalysts for reaction (1).20,21 We employed STEM/HAADF (High Angle Annular Dark Field) and STEM/EELS (Electron Energy Loss Spectroscopy) techniques together with XPS (X-ray Photoelectron Spectroscopy), XRD (X-ray Diffraction), and ICP (Inductive Coupling Plasma), to give a structural and chemical view of the Co–B–O material and Co–Ru–B–O series at the micro and nanoscale.20,21 These materials have shown a complex nanostructure characterized by the formation of very small metallic nanoparticles embedded in an amorphous matrix. The Co–B–O material contains Co nanoparticles embedded in a matrix composed of CoxB (cobalt boride), B2O3 and Co–B–O amorphous phases. The presence of interstitial boron in Co nanoparticles was proposed.20 For the Co–Ru–B–O series, the Co-containing samples, present a microstructure composed of 20–40 nm size grains which contain tiny metallic (Co–Ru) nanoparticles embedded in an amorphous matrix constituted by Co–Ru–B–O phases. A veil of Co(BO2)2 is surrounding all the structure which tends to decrease in thickness and coverage degree with Ru content. For the Ru–B (xRu = 1) sample there is a drastic decrease in boron content which produces a change in nanostructure characterized by an abrupt increase in particle size respect to the rest of the series. In that paper, we proposed a structural model that depicted a representation of the nanostructure and composition of the series.21 This structural model was adequate for a qualitative explanation of the enhancement in catalytic activity of the Co–Ru–B–O powders respect to the monometallic Co–B–O and Ru–B–O materials.21 However, some questions related to these materials remain unanswered. The study of metal nanoparticle size, chemical composition of the matrix in the nanoscale, and the formation or not of a CoxRu1−x alloy or solid solution was not done before. These studies could be very important for a full comprehension of catalytic properties. Furthermore, the magnetic properties of these nanoalloys are interesting and were not studied before. For these reasons, in this work the Co–Ru–B–O series was deeply studied employing STEM techniques together with XAS (X-Ray Absorption Spectroscopy) for a full chemical and structural characterization. Also magnetic properties of these materials were studied for the first time. Nanostructure, composition, electronic structure and magnetism are correlated and discussed along the Co–Ru–B–O series by the integration of these characterization techniques giving new insights into the study of the cobalt–ruthenium–boron interactions in the nanoscale.
2. Experimental
2.1 Sample preparation
Ultrafine Co–Ru–B–O powder materials were prepared as previously by chemical reduction of an aqueous solution of CoCl2·6H2O and RuCl3·3H2O with variable xRu (where xRu is considered as nmol Ru/(nmol Ru + nmol Co)) by aqueous sodium borohydride (NaBH4).21Cobalt borate (Co(BO2)2) reference sample was obtained by precipitation of a BO2− containing aqueous solution with CoCl2·6H2O as previously reported in.20 Cobalt boride (CoxB x = 1, 2) and RuO2 reference samples were purchased from Sigma Aldrich and used as received.
2.2 Nanostructural and chemical characterization
For the Electron Microscopy studies, powder samples were impregnated on a copper grid coated with a holey-carbon film. The studies were performed using several microscopes.A JEOL JEM 2010F Scanning electron transmission FEG (Field Emission Gun) microscope, equipped with a HAADF detector and an imaging filter from Gatan GIF2000. For the Spectrum Imaging (SI) mode, a 0.5 nm beam with a current of 0.1–0.3 nA scanned along regions of the sample. The HAADF signal was also simultaneously collected at each point within the scanned region.
A FEI FEGTEM Tecnai G2 F30 S-Twin, equipped with a HAADF detector from Fischione Instruments, an SDD X-Max energy-dispersive X-ray spectrometer (EDXS) detector from Oxford Instruments and an image filter Quantum 96 from Gatan.
Samples for 3D characterization by Electron Tomography were prepared by depositing a small amount of the catalyst powder onto a holey carbon film supported by a 300 mesh copper tomography grid. Tomographic tilt-series in HAADF-STEM mode were acquired using a JEOL JEM 2010F electron microscope operated at 200 kV using a Fischione Ultra-Narrow Gap tomography holder. Series of images of Ru–Co–B (xRu = 0.13) sample were acquired over a wide angular range (from −70° to +70°) at tilt increment of 2° using a magnification of 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times. After data acquisition all images were aligned with respect a common origin and tilt axis using Inspect 3D software (FEI). In the next step 3D reconstructions were computed using the simultaneous iterative reconstruction technique (SIRT), which constrains the reconstructed volume to best match the original images when reprojected back along the original tilt directions. Voxel projections and surface rendering were undertaken using Amira 3.1 software.
000 times. After data acquisition all images were aligned with respect a common origin and tilt axis using Inspect 3D software (FEI). In the next step 3D reconstructions were computed using the simultaneous iterative reconstruction technique (SIRT), which constrains the reconstructed volume to best match the original images when reprojected back along the original tilt directions. Voxel projections and surface rendering were undertaken using Amira 3.1 software.
XAFS measurements were performed at the Swiss Norwegian Beam Line (SNBL-BM01B) of the European Synchrotron Radiation Facility (ESRF, Grenoble, France). The spectra were obtained at room temperature at the Co K-edge (7709 eV) and Ru K-edge (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 117 eV) in the transmission mode with cellulose dilution. Co and Ru metal foil were used as reference for energy calibration. Reference RuO2, Co(BO2)2, cobalt boride (CoxB) and metal foils were used as standards for oxidation state estimation. Data treatment was performed with ATHENA and ARTHEMIS codes.22
117 eV) in the transmission mode with cellulose dilution. Co and Ru metal foil were used as reference for energy calibration. Reference RuO2, Co(BO2)2, cobalt boride (CoxB) and metal foils were used as standards for oxidation state estimation. Data treatment was performed with ATHENA and ARTHEMIS codes.22
Magnetic measurements were performed using a Quantum Design SQUID Magnetometry. The diamagnetic contribution corresponding to the sample holder system has been previously measured. The samples were measured as powders, slightly compacted inside the sample holder.
3. Results
3.1 Nanostructure and particle size
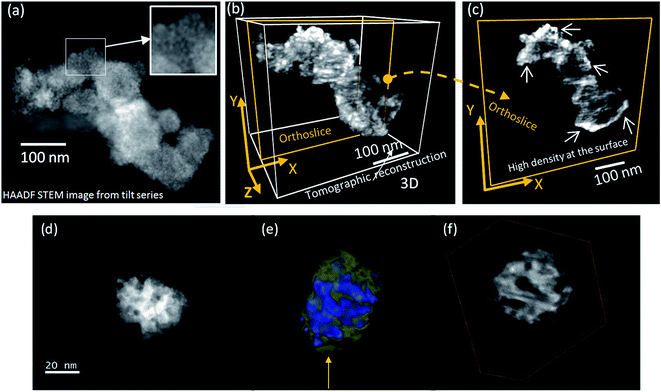
Fig. 1 shows a representative TEM micrograph and a graphic representation of the nanostructure and composition of the Co–Ru–B–O series (except for Ru–B–O sample) as we previously reported.21 Samples show a complex nanostructure in which phases with different density are involved. For this type of system the use of HAADF-STEM imaging technique is very useful, because signal intensity is proportional to Z3/2t (where Z is the atomic number and t is sample thickness). These also-called Z-contrast images permit to distinguish the nanoparticles from the low Z matrix. Moreover, by using a series of HAADF-STEM images recorded at different tilt angles from −70° to 70°, an electron tomogram can be obtained to get information about 3D nanostructure and phases' distribution.23 Fig. 2a shows a representative Z-contrast image of the xRu = 0.13 sample acquired at 200 kV. Small nanoparticles can be seen (inset in Fig. 2a) also forming bigger spherical 20–40 nm particles. For the rest of the samples, Z-contrast images show a similar nanostructure. Fig. 2b shows a tomographic reconstruction (volume of ∼500 nm3) obtained from the tilt series of images such as that shown in (a). The voltex visualisation of the three-dimensional shape was optimized to fit the outer shape. In Fig. 2c is presented an orthoslice extracted from the reconstructed volume shown in Fig. 2b. The intensities of the orthoslice, which are proportional to the local density of the sample, show that density is inhomogeneous. Image intensities are brighter on surface (see white arrows) in comparison to the darker intensities inside suggesting that Ru accumulates on surface. A more detailed Electron Tomography study is performed on a spherical grain of around 30 nm diameter size and is shown in Fig. 2d (below). The reconstructed volume is shown in Fig. 2e, where low Z zones are colored in green (veil), high Z (metallic) nanoparticles are in brilliant blue, and matrix is in dark blue. An internal mesoporous structure is found with a pore diameter of ca. 5 nm. The internal porosity is evident from the axial slice through the reconstruction displayed in Fig. 2f. A movie (in mpg format as ESI†) showing the full dynamic tomogram is available as a web enhanced object, where the presence of the veil is also very clear. It is clear from the reconstruction that small particles are completely embedded within the porous matrix. | ||
| Fig. 1 TEM image and graphic showing a general representation of the nanostructure and composition of the Co–Ru–B–O series (except for Ru–B–O sample) as reported in ref. 21. | ||
The study of particle size is a difficult and controversial task for this type of materials because the involved phases are amorphous or nano-crystalline. However, the estimation of particle size is essential to understand catalytic and magnetic behavior. High Resolution Electron Microscopy (HREM) is well known to provide information about particle size and crystalline phases. The series was studied by HREM but unfortunately samples were not stable under the electron beam, changing in size and crystallizing. Instead, the intensity profile (signal intensity as a function of probe position) of several Z-contrast images has been analyzed to determine an approximate size of the brighter cores, corresponding to Co/Ru rich nanoparticles. Fig. 3 shows the variation the particle size as a function of Ru content. Average size is almost constant and around 1.5 nm for the Co-containing materials and increases to 3 nm for the Ru–B–O sample.
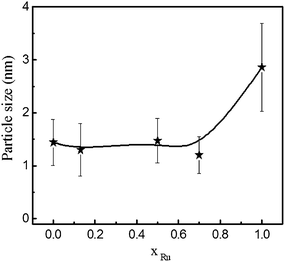 | ||
| Fig. 3 Particle size determined by the analysis of several STEM/HAADF images as a function of Ru content. | ||
3.2 Chemical characterization
Due to the amorphous character of the matrix and the small size of the nanoparticles embedded in it, the study of involved phases requires the use of XAS and EELS techniques. Both techniques permit to study electronic states: EELS gives information with lateral resolution while XAS senses an average of the whole sample. In this sense both EELS and XAS are complementary.The XANES (X-ray absorption near edge structure) region of the XAS spectra contains information of oxidation states and provides electronic information of the absorbing atom and its environment. Although multiple scattering calculations can be carried out to simulate the XANES spectra, a fingerprinting method can be used by the comparison of results with reference samples.24 Fig. 4 a shows the Co K-edge for the Co–Ru–B–O series in comparison with references (Co foil, Co(BO2)2 and cobalt boride). The spectra show two remarkable features labeled A (∼7713 eV) and B (∼7725 eV) with different nature.24,25 The A feature, or pre-edge, has a low intensity because of its forbidden nature (a d-level as final state). The B transition has a p-level as final state and is normally called “white line”. Meitzner et al. related the white line intensity in the 5d metal series with the empty states above the Fermi level, showing that the white line decreases when less empty levels are available.26 Also Hlil et al. described a reciprocal relationship between the pre-edge and the white line along the series of variable composition for the Co/Pt system.27 In our case, by comparison the spectra of the series with references, a decrease in the intensity of A and an increase of B is observed with Ru content. These features indicate a change in the empty levels available with respect Co foil. That could be explained by the presence of Co–B–O and Co–Ru based phases. The comparison of E0 along the series (obtained from the derivative of the Co K edge, Fig. 4b) shows that most Co remains in metallic state, independently of Ru content.
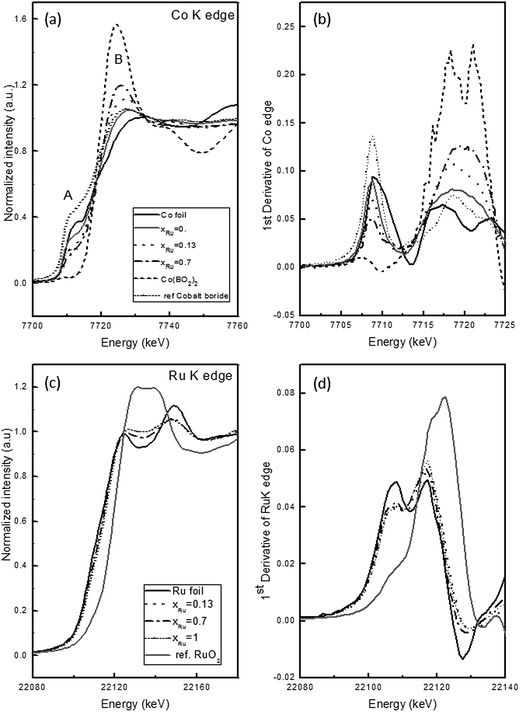 | ||
| Fig. 4 XANES spectra on the Co–Ru–B series in comparison with references (a) Co K-edge (b) derivate of Co K-edge (c) Ru K-edge (d) derivate of Ru K-edge. | ||
The Ru K-edge XANES spectra are plot together with Ru foil and RuO2 as reference samples in Fig. 4c. The comparison of prepared samples with Ru foil shows similar features but less intense. Similar results were reported by O'Grady et al., indicating that Ru K-edge is not so sensitive to changes in alloys with other metals.28 Also it is clearly observed that these features are damped in the NPs samples respect to the metal foil. This effect is due to structural disorder in the samples.29 The comparison of E0 (Fig. 4d) shows that along the series, Ru is mostly present in metallic state as occurs with Co.
As mentioned above, XAS provides “structure-average” information of the same absorber atom. If the absorber atoms adopt several configurations, individual local structural information is obtained as a weighted average of all configurations. In this case EELS studies are essential to get chemical information in the nanoscale. The study of ELNES (Electron Energy Loss Near Edge Structure) can provide information on the local coordination and oxidation states. The use of STEM mode with small probe size (less than 1 nm) permits to get this information with a very high lateral resolution.30 Furthermore, this technique is especially suitable for the characterization of light elements. The use of EDX measurements in parallel with EELS in STEM mode permits also to get elemental maps by suitable quantification of the spectra.
For the xRu = 0 sample, the ELNES study of the B–K, O–K and Co–L2,3 edges in comparison with references (finger-printing) permitted previously the elucidation of composition in the nanoscale.20 Similar methodology was employed in this work to study the elemental distribution and chemical composition and of the rest of Co-containing materials of the series. For these samples EELS and EDX spectra were simultaneously measured with high lateral resolution and B, O, Co and Ru maps were obtained using the Spectrum-Imaging method.30 Fig. 5 shows the maps obtained for the xRu = 0.13 sample. Co elemental map shows a heterogeneous distribution throughout the area, with presence of nanoparticles very rich in it. The Ru map also shows cores very rich in ruthenium located preferentially on surface, in agreement with the tomography result. In both maps, small metallic nanoparticles are well distinguished from the matrix and the approximate size obtained by the study of HAADF images (section 3.1) is confirmed. Oxygen and boron maps show a heterogeneous distribution of both elements. Elemental maps obtained for the xRu = 0.7 sample (not shown), indicate also a heterogeneous distribution of the elements in the nanoscale.
 | ||
| Fig. 5 EDX and EELS elemental maps obtained on a 20 × 20 nm2 marked zone of the xRu = 0.13 sample using the SI method. | ||
To get information about the phases in the nanoscale, EELS spectra measured along 16 nm lines using a 0.5 nm probe size were studied. B and O K-edge, Ru M4,5-edge, and Co L2,3-edges were recorded for the xRu = 0.13 and xRu = 0.7 samples. O–K edges, not shown, presented low signal intensity. Representative spectra on five positions (a–e; f–j) are shown in Fig. 6. The study of the B–K edge on both samples (Fig. 6a and c) and the comparison with edges measured for reference materials from our previous work shows contributions of CoxB (cobalt boride), B2O3, Co–B–O phases.20 For the xRu = 0.13 sample there is a major contribution of CoxB (points a, b and d), with minor presence of B2O3, and Co–B–O (points c and d respectively). The shape of B–K edge in point a, b and d, is also consistent with the presence of interstitial B.20 On the contrary, for the xRu = 0.7 sample there is a high contribution of Co–B–O (points f, h, i) and CoxB and B2O3 (points j and g respectively) in a lesser extent. The shape of Ru M4,5-edges was compared with reference samples studied in another paper.21 These edges are typical of metallic state for both samples (Fig. 6b and d) with a small contribution of oxidized ruthenium.
 | ||
| Fig. 6 Selected EELS spectra from different points on a 16 nm scanned line along the xRu = 0.13 and 0.7 samples, (a), (c) B K-edge (b), (d) Ru M4,5-edge. | ||
The intensities of Co-L2,3 edges resonances (white line); correspond to a dipolar transition (2p1/2 → 3d and 2p3/2 → 3d) which reflect the occurrence of unoccupied states in the d band. The L3/L2 area ratio depends on chemical composition, structure, formation of alloys, crystal field, particle size, and magnetic moment.31 For this reason, the exact interpretation of the L3/L2 ratio is a difficult task. As an approximation L3/L2 measurements can be compared with those obtained with reference materials to get an idea of the oxidation state as we did in a previous paper.20 In this work this ratio was approximated by the ratio of intensities of the L2 and L3 white lines. In Fig. 7 the ratio was calculated as a function of the probe position. For the xRu = 0.13 sample, a L3/L2 = 1.4 was obtained (at 7 nm) indicating that cobalt is in reduced form, as Co0 or CoxB. Despite this, most positions show a ratio around 1.5. This could indicate presence of cobalt in Co–B–O and Co–Ru phases. However, the analysis of B K-edges indicates low contribution of B–O. For this reason, the increase in the L3/L2 can be explained as due to the presence of Co–Ru phases with a small contribution of Co(BO2)2 on surface. For the xRu = 0.7 sample, most points show a L3/L2 ratio around 1.75, in the range of cobalt oxides (CoO and Co3O4). The study of B and O K-edges showed that oxygen is preferentially bound to boron. Furthermore, for this sample the contribution of Co(BO2)2 phases is negligible, as previously shown in our previous XPS measurements, which means that the presence of some Co–O bonds forming CoO cannot be disregarded.21 In this sample the increase of the L3/L2 ratio could be attributed both to the presence of some CoO and to the formation of Co–Ru bonds. The presence of different chemical environments for cobalt, one with a metallic character (Co, Co–Ru, CoxB) and other oxidized (Co(BO2)2, CoO) are in agreement with the trend observed in the Co–K edge along the series (Fig. 4).
 | ||
| Fig. 7 Co L3/L2 ratio as a function of probe position on a 16 nm scanned line along the xRu = 0.13 and 0.7 samples. | ||
3.3 Formation of Co/Ru solid solutions
Co–Ru bulk phase diagram was studied previously and shows that at room temperature, Co and Ru form solid solutions for the whole range of composition.32 Ru incorporates in the hcp Co lattice and the lattice parameters follow a linear relationship with Ru content. The behavior of Co–Ru samples in the nanoscale does not necessarily obey the phase diagram but it gives an idea of the type of interactions between both atoms. For the herein discussed materials, the study of the formation or not of a solid solution is not simple. Structure and composition vary in the nanoscale as demonstrated by EELS and EDX elemental maps (Fig. 5) discussed in previous sections. Furthermore, changes observed in the Co K and L2,3 edges for the Ru containing samples would be consistent with the formation of Co–Ru bonds. To test the hypothesis of the formation Co–Ru solid solutions (denoted as CoxRu(1−x)), the EXAFS region of the XAS spectra was analyzed. From the fitting of the EXAFS oscillations, information about the neighboring atoms, coordination number and distances between neighbors can be obtained. Tables 1 and 2 show the parameters (distances and coordination numbers respectively) obtained from the fitting of the EXAFS oscillations from Co and Ru K-edges. Metal-boron and metal-oxygen distances are indistinguishable with this technique due to the closer atomic number and similar atomic factors. The distances are similar for Ru–(B/O) and Co–(B/O) (∼2 Å) independently of composition. As the first shell can be composed by three elements, O/B, Co and Ru, the inclusion of all these parameters increases the uncertainties in the results. This situation is more favorable for Ru because the k-space region to fit is larger than Co case. Even though the uncertainties vary from Co to Ru, the intervals overlap. The coordination numbers (CN) in Table 2 exhibit a drastic reduction respect to bulk results. This result can be explained due to disorder and surface effects.33 The amorphous/nanocrystalline character of samples and the appearance of dangling bonds on such small nanoparticles (with high surface to bulk atoms ratio) contribute to the drastic decrease in CN.| Co K-edge | Ru K-edge | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d Co–Co | d Co–Ru | d Co-(O/B) | d Ru–Co | d Ru–Ru | d Ru-(O/B) | |||||||
| Distance | Error | Distance | Error | Distance | Error | Distance | Error | Distance | Error | Distance | Error | |
| xRu = 0 | 2.477 | 0.025 | 1.933 | 0.046 | ||||||||
| xRu = 0.13 | 2.548 | 0.061 | 2.584 | 0.134 | 1.988 | 0.081 | 2.572 | 0.010 | 2.647 | 0.008 | 2.000 | 0.013 |
| xRu = 0.7 | 2.511 | 0.039 | 2.572 | 0.010 | 2.042 | 0.089 | 2.627 | 0.021 | 2.737 | 0.022 | 2.014 | 0.030 |
| xRu = 1 | 2.660 | 0.005 | 1.987 | 0.027 | ||||||||
| Co K-edge | Ru K-edge | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CN Co–Co | CN Co–Ru | CN Co-(O/B) | CN Ru–Co | CN Ru–Ru | CN Ru-(O/B) | |||||||
| CN | Error | CN | Error | CN | Error | CN | Error | CN | Error | CN | Error | |
| xRu = 0 | 4.6 | 1.5 | 1.7 | 0.9 | ||||||||
| xRu = 0.13 | 1.6 | 1.9 | 1.0 | 2.2 | 1.8 | 1.4 | 1.5 | 0.3 | 4.9 | 0.5 | 1.7 | 0.3 |
| xRu = 0.7 | 1.0 | 1.8 | 1.1 | 2.1 | 2.9 | 2.7 | 2.7 | 0.8 | 2.2 | 0.4 | 0.9 | 0.3 |
| xRu = 1 | 5.8 | 0.6 | 1.5 | 0.4 | ||||||||
3.4 Magnetic behaviour
Fig. 8, left shows the thermal dependence of the magnetization (M) measured with a 5 T applied field for the Co–Ru–B series. The xRu = 1 sample exhibits a diamagnetic behavior with no change in Ms (saturation magnetization) with temperature, while the rest of the series (Co-containing samples) exhibit a ferromagnetic behavior. For the latter, the study of the derivate of these curves (Fig. 8, right), indicates the presence of two magnetic phases: one with a low Curie point (below 50 K for samples xRu 0, 0.13 and 0.5 and around 15 K for sample xRu 0.7), and other that exhibits a ferromagnetic behavior up to room temperature. The ferromagnetic behavior is also evidenced by the hysteresis loops (at different temperatures) shown in Fig. 9a for the xRu 0.13 sample. All the Co-containing samples show hysteresis loops up to room temperature and magnetization does not even saturate for fields up to 5 T. The plot of the Hc (coercitive field) as a function of temperature (Fig. 9b) supports the hypothesis of the two magnetic phases. Hc exhibits a sharp decrease from 5 to 50 K and an almost linear dependence with temperature. | ||
| Fig. 8 Left: magnetization as a function of temperature for the Co–Ru–B series with a 5 T field. Right: derivative of magnetization with respect to T, as a function of temperature. | ||
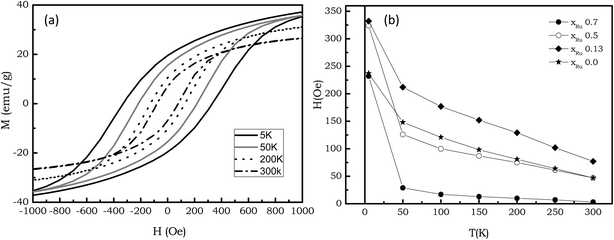 | ||
| Fig. 9 (a) Hysteresis loop for the xRu = 0.13 sample (b) Coercitive field as a function of temperature for the series. | ||
Fig. 10a and b show the variation of Hc and Ms (saturation magnetization) respectively at 5 and 300 K with the Ru content. The addition of a small amount of Ru (xRu = 0.13) produces an increase in the Hc respect to Co–B–O sample, which indicates stronger anisotropy fields. Also there is an increase in the Ms from xRu 0 to xRu 0.13. Further increase in Ru content promotes a decrease in Hc and Ms.
 | ||
| Fig. 10 (a) Coercitive field and (b) saturation magnetization (per gram of Co) as a function of Ru content. | ||
3.5 Nanoparticles, amorphous matrix and ferromagnetic behavior: structure–performance relationship
Magnetic measurements reveal the presence of a complex magnetic structure in which phases with different behavior coexist. For such system magnetic behavior depends not only on the individual components but also on the interaction between the different phases, i.e. particle–particle interactions transmitted by the matrix, and particle–matrix interactions.As discussed in previous sections, for the Co containing samples of the series, the structure is characterized by the formation of 20–40 nm particles which include even smaller (2 nm) metallic nanoparticles embedded in an amorphous matrix of CoxB (cobalt borides), B2O3, cobalt oxides and Co–Ru–B–O phases. All the structure is surrounded by a Co(BO2)2 veil. B2O3 and Ru–O phases are non-magnetic. Co oxides are antiferromagnetic in bulk, with a Neel temperature (TN) is 291 K and 40 K for CoO and Co3O4, respectively. Co3B and Co2B phases are ferromagnetic (Curie temperatures of 747 K and 433 K, respectively) and an amorphous Co–B alloy has been also reported to be ferromagnetic at room temperature.34 For this reason CoxB phases are also expected to be ferromagnetic.
As concerns to the small (less than 2 nm) nanoparticles, they are mainly composed of Co, CoxRu(1−x) or Ru. The latter is non-magnetic, therefore its contribution should not be considered. Co is a well-known ferromagnetic material with 1388 K Curie temperature. Isolated 2 nm Co nanoparticles are superparamagnetic at temperatures around 10 K.35 Co–Ru nanoparticles (2 nm) have been reported to be ferromagnetic below 2 K with high values of the coercive field, (1000–8000 Oe), whereas a superparamagnetic behavior is observed above this temperature.36
In our case, Co–Ru–B–O samples exhibit ferromagnetism up to room temperature, with coercive field values ranging between 50 and 80 Oe. Taking into account the small size of the metal-rich nanoparticles, the origin of this behavior arises mainly from CoxB phases in the matrix. By decreasing the temperature to 50 K, the coercive field increases as expected for a ferromagnetic material. However, by a further decrease to 5 K a drastic hardening is observed. This behavior should be attributed to Co or CoxRu(1−x) nanoparticles that are blocked at 5 K. By increasing the temperature, these nanoparticles become unblocked and enter into the superparamagnetic regime giving no contribution to the hysteresis.
The evolution of Hc and Ms with the composition shows that a small addition of Ru increases the anisotropy field, as evidenced by the increase of the coercive field observed at low temperatures in samples xRu 0.13 (Fig. 10a). This hardening can be explained by the formation of a CoxRu(1−x) solid solution. The increase in the Ru content promotes a softening that is especially evident in the case of xRu = 0.7 sample. Also higher values of the high field susceptibility are obtained. Simultaneously the magnetization decreases. The above can be interpreted by considering two magnetic contributions from the matrix, CoxB and CoO. For sample xRu = 0.7 hysteresis loops for temperatures above 50 K shows low values of the coercive field, being close to zero at room temperature. This behavior can be explained taking into account that the matrix is mainly composed by a CoO antiferromagnetic phase. As it is well known antiferromagnetic materials are characterized by low magnetization values in combination with high values of the high field susceptibility. The small hysteresis could arise from some clustering of nanoparticles as well as from the CoxB remaining phase. By reducing Ru content (xRu = 0.5 and xRu = 0.13), the increase magnetization values as well as a decrease of the high field susceptibility are explained by the increase in the amount of the CoxB with respect to CoO.
4. Conclusions
A series Co–Ru–B–O ultrafine materials with variable Ru content previously employed as catalysts for hydrogen generation, were exhaustively studied using microscopic and spectroscopic techniques (HAADF-STEM, electron tomography, EDX, and EELS, with high lateral resolution, and also XAS) and magnetically. Despite the complexity of these materials, the study confirms our previous hypothesis of the formation of very small metallic nanoparticles (Co, Ru and CoxRu1−x) of less than 2 nm size, embedded in an amorphous porous matrix, mainly composed of CoxB, and B2O3, Co–O and Co–Ru–B–O phases. The ferromagnetic character at room temperature of the Co-containing samples can be explained by the presence of CoxB amorphous phases in the matrix. The hardening observed for T < 50 K is due to the presence of metallic magnetic nanoparticles that are blocked. The addition of Ru to the Co–B–O sample produces also an hardening at low temperature that confirm the formation of CoxRu(1−x) solid solution, as suggested by the analysis of the EELS and XAS and spectra. Further addition of Ru produces a change in the matrix composition characterized by the reduction of the amount of CoxB and the formation of CoO, an antiferromagnetic phase that explains the magnetic behavior of the xRu = 0.7 (lower Hc and high field susceptibility). As a consequence of this study, the issues raised related to structure, chemical composition, and the formation of solid solution were solved, contributing to the comprehension of the nature of metal–metalloid interactions in these controversial nanoalloys.Acknowledgements
Financial support from MINECO (CTQ2012-32519), CSIC (PIE 201260E006 and 201060E102), Junta de Andalucía (TEP217, PE2012-TEP862) the EC (CT-REGPOT-2011-1-285895, AL-NANOFUNC) and MAT2012-37109-C02-01 is acknowledged. XAFS experiments were performed on the Swiss-Norwegian Beam Line (SNBL-BM01B) at the European Synchrotron Radiation Facility (ESRF), Grenoble, France. We are grateful to Paula Abdala at ESRF for providing assistance during the measurements (proposal 01-01-894). Authors are also very grateful to Victor Velasco for SQUID measurements.References
- A. Züttel, A. Borgschulte and L. Schlapbach, in Hydrogen as a Future Energy Carrier, Wiley-VCH, 2008 Search PubMed
.
- L. Schlapbach and A. Züttel, Nature, 2001, 414, 353–358 CrossRef CAS PubMed
.
- G. Moussa, R. Moury, U. B. Demirci, T. Sener and P. Miele, Int. J. Energy Res., 2013, 37, 825–842 CrossRef CAS
.
- B. H. Liu and Z. P. Li, J. Power Sources, 2009, 187, 527–534 CrossRef CAS PubMed
, references therein.
- U. B. Demirci, O. Akdim, J. Andrieux, J. Hannauer, R. Chamoun and P. Miele, Fuel Cells, 2010, 3, 335–350 CrossRef
, references therein.
- S. S. Muir and X. Yao, Int. J. Hydrogen Energy, 2011, 36, 5983–5997 CrossRef CAS PubMed
, references therein.
- E. Y. Marrero-Alfonso, J. R. Gray, T. A. Davis and M. A. Matthews, Int. J. Hydrogen Energy, 2007, 32, 4723–4730 CrossRef CAS PubMed
.
- C. F. Lo, K. Karan and B. R. Davis, Ind. Eng. Chem. Res., 2007, 46, 5478–5484 CrossRef CAS
.
- U. B. Demirci, O. Akdim, J. Andrieux, J. Hannauer, R. Chamoun and P. Miele, Sci. China: Chem., 2010, 53, 1870–1879 CrossRef CAS
, references therein.
- U. B. Demirci and P. Miele, Phys. Chem. Chem. Phys., 2010, 12, 14651–14665 RSC
, references therein.
- U. B. Demirci and P. Miele, Phys. Chem. Chem. Phys., 2014, 16, 6872–6885 RSC
.
- O. Akdim, U. B. Demirci, D. Muller and P. Miele, Int. J. Hydrogen Energy, 2009, 34, 2631–2637 CrossRef CAS PubMed
.
- H. Li, J. Liao, X. Zhang, W. Liao, L. Wen, J. Yang, H. Wang and R. Wang, J. Power Sources, 2013, 239, 277–283 CrossRef CAS PubMed
.
- O. Akdim, R. Chamoun, U. B. Demirci, Y. Zaatar, A. Khoury and P. Miele, Int. J. Hydrogen Energy, 2011, 36, 14527–14533 CrossRef CAS PubMed
.
- N. Patel, A. Miotello and V. Bello, Appl. Catal., B, 2011, 103, 31–38 CrossRef CAS PubMed
.
- O. V. Komova, V. I. Simagina, O. V. Netskina, D. G. Kellerman, A. V. Ishchenko and N. A. Rudina, Catal. Today, 2008, 138, 260–265 CrossRef CAS PubMed
.
- N. Patel, R. Fernandes and A. Miotello, J. Power Sources, 2009, 188, 411–420 CrossRef CAS PubMed
.
- S. Carenjco, D. Portehault, C. Boissière, N. Mézailles and C. Sanchez, Chem. Rev., 2013, 113, 7981–8065 CrossRef PubMed
.
- Y. Pei, G. Zhou, N. Luan, B. Zong, M. Qiao and F. Tao, Chem. Soc. Rev., 2012, 41, 8140–8162 RSC
.
- G. M. Arzac, T. C. Rojas and A. Fernández, ChemCatChem, 2011, 3, 1305–1313 CrossRef CAS
.
- G. M. Arzac, T. C. Rojas and A. Fernández, Appl. Catal., B, 2012, 128, 39–47 CrossRef CAS PubMed
.
- B. Ravel and M. Newville, J. Synchrotron Radiat., 2005, 12, 537–541 CrossRef CAS PubMed
.
- L. C. Gontard, R. E. Dunin-Borkowski and D. Ozkaya, J. Microsc., 2008, 232, 248–259 CrossRef CAS PubMed
.
- A. Bianconi, X-Ray Absorption: Principles, Applications, Techniques of EXAFS, SEXAFS and XANES, ed. D. C. Konigsberger and R. Prins, John Wiley, New York, 1988, ch. 11, pp. 573–662 CrossRef
; H. Kisker, T. Gessman, R. Wurschum, H. Kronmüller and H. E. Schaefer, Nanostruct. Mater., 1995, 6, 925–928 CrossRef
.
- T. C. Rojas, J. C. Sánchez-López, M. J. Sayagués, E. P. Reddy, A. Caballero and A. Fernández, J. Mater. Chem., 1999, 9, 1011–1017 RSC
.
- G. Meitzner, G. H. Via, F. W. Lytle and J. H. Sinfelt, J. Phys. Chem., 1992, 96, 4960–4964 CrossRef CAS
.
- E. K. Hlil and R. Baudoing-Savois, J. Phys. Chem., 1996, 100, 3102–3107 CrossRef CAS
.
- W. E. O'Grady, P. L. Hagans, K. I. Pandya and D. L. Maricle, Langmuir, 2001, 17, 3047–3050 CrossRef
.
- J. J. Rerhr and A. L. Ankudinov, Coord. Chem. Rev., 2005, 249, 131–140 CrossRef PubMed
.
- C. Jeanguillaume and C. Colliex, Ultramicroscopy, 1989, 28, 252 CrossRef
.
- C. Colliex, T. Manoubi and C. Ortiz, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 44(20), 11402–11411 CrossRef CAS
.
- W. Köster and E. Horn, Z. Metallkd., 1952, 43(12), 444–449 Search PubMed
.
- T. Haubold, F. Boscherini, S. Pascarelli, S. Mobilio and H. Gleither, Philos. Mag. A, 1992, 66, 591 CrossRef CAS
.
- A. B. Davila-Ibánez, J. L. Legido-Soto, J. Rivas and V. Salgueirino, Phys. Chem. Chem. Phys., 2011, 13, 20146–20154 RSC
.
- M. Respaud, J. M. Broto, H. Rakoto, A. R. Fert, L. Thomas, B. Barbara, M. Verelst, E. Snoeck, P. Lecante, A. Mosset, J. Osuna, T. Ould Ely, C. Amiens and B. Chaudret, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 2925 CrossRef CAS
.
- D. Zitoun, C. Amiens, B. Chaudret, M. C. Fromen, P. Lecante, M. J. Casanove and M. Respaud, J. Phys. Chem. B, 2003, 107, 6997–7005 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05700g |
| This journal is © The Royal Society of Chemistry 2014 |