Processing of plasma-modified and polymer-grafted hydrophilic PET surfaces, and study of their aging and bioadhesive properties
Abstract
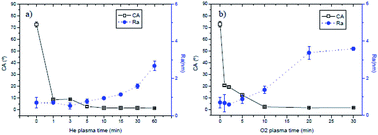
In this work, poly(ethylene terephthalate) (PET) films were treated by oxygen and helium plasmas and their chemistry and morphology were studied. Samples were characterized by X-ray photoelectron spectroscopy (XPS), atomic force microscopy (AFM), scanning electron microscopy (SEM) and water contact angle (WCA) measurements. The aging of plasma-treated PET films was studied in different media (air and water) by WCA. The anti-fouling properties of the plasma treated surfaces were evaluated by confocal microscopy. Both oxygen and helium plasma-treatments produced hydrophilic and nano-structured surfaces that presented a remarkable reduction of the bioadhesive character. Besides, the grafting of plasma treated surfaces was explored using Pluronic F108 in order to improve the anti-fouling properties of the plasma treated surfaces.

 Please wait while we load your content...
Please wait while we load your content...