Environment-resistant fluoro-containing antireflective coatings for high-powered laser systems
Xinxiang Zhang*a,
Mengyun Zhuanga,
Xia Miaod,
Wenmu Sua,
Mingyue Lina,
Lixiao Lina,
Longqiang Yeb,
Lianghong Yanc,
Wenbin Yanga and
Bo Jiangb
aCollege of Materials Engineering, Fujian Agriculture and Forestry University, Fuzhou, 350002, China. E-mail: xxzhang0106@163.com; Fax: +86-951-83715175; Tel: +86-951-83715175
bKey Laboratory of Green Chemistry & Technology, College of Chemistry, Sichuan University, Chengdu, 610064, China
cResearch Center of Laser Fusion, China Academy of Engineering Physics, Mianyang, 621900, China
dSINOPEC Research Institute of Petroleum Engineering, Beijing, 100101, China
First published on 3rd September 2014
Abstract
A simple sol–gel route was proposed to prepare environment-resistant fluoro-containing antireflective (AR) coatings with nearly 100% transmittance by co-condensation of tetraethylorthosilicate and 1H,1H,2H,2H-perfluoroalkyltriethoxysilanes (POTS). These AR coatings can find great applications in high-powered laser systems.
High-powered laser systems were designed to initiate a fusion reaction and gain a large amount of energy with no greenhouse gas emissions and less hazardous radioactive byproducts than current fission power plants. The most powerful high-powered laser system is the national ignition facility (NIF), which contains 7360 meter-scale optics, including hundreds of transmissive optics.1 The refractive indices of the transmissive optics in the NIF are 1.46–1.52. According to the Fresnel equation, there is about 8% reflection for each transmissive optic. Reflection on the surface of hundreds of transmissive optics will significantly decrease the energy of lasers directed into the target that were used to initiate the fusion reaction. It is very helpful to suppress the maximum reflection of the transmissive optics with an antireflective (AR) coating.1,2 The refractive index of sol–gel silica AR coating is equal to the square root of that of many common optical glasses, giving the coated glasses nearly 100% transmission.3 In addition, the laser-induced damage threshold (LIDT) of sol–gel silica AR coating is two to three times higher than that of AR coatings from the physical vapor deposition method. Therefore, sol–gel silica AR coatings have been widely used on transmissive optics in the NIF.4
However, sol–gel silica AR coatings are hydrophilic and possess high specific surface areas, and therefore tend to adsorb contaminants, such as water or polar organic molecules (plasticizers of engineering materials in the NIF), from the user environment.5 The contaminants adsorbed into the pores of the AR coatings degrades the optical property, which is known as the poor environmental resistance of the hydrophilic silica AR coating. To overcome this problem, great efforts have been devoted to prepare ORMOSIL (organically modified silicate) AR coatings. In ORMOSIL AR coatings, there are many organic hydrophobic groups, which can hinder the coating from adsorbing polar pollutants. The most common methods for preparation of ORMOSIL AR coatings are as follows: (1) surface modification of AR coatings;6,7 (2) co-condensation of tetraethylorthosilicate (TEOS) and hydrophobic silane precursors (e.g. methyltriethoxysilane (MTES) or dimethyldiethoxysilane (DDS));8,9 and (3) hybrids of silica with polymers.10,11 However, to achieve satisfiable environmental resistance, these methods need to add large amounts of polymer or hydrophobic silane precursors to give the AR coatings enough hydrophobic groups. This affects the microstructure of the silica clusters and the stacking texture of the AR coatings, which reduces the transmittance.9 In the NIF, the transmittance should be higher than 99.5%. 1H,1H,2H,2H-Perfluoroalkyltriethoxysilanes (POTS) is a very effective hydrophobic modifier due to the low surface energy of its perfluoroalkyl chains.12 In this work, we prepared environment-resistant fluoro-containing ORMOSIL AR coatings with nearly 100% transmittance by co-condensation of TEOS with a small amount of POTS.
TEOS, POTS, isopropanol, and ammonia were added in a sealed glass container and then immediately stirred for 2 hours at 30 °C. The chemical formulas are shown in Table 1. The content of POTS was adjusted to give ORMOSIL sols with different fluorine contents. The sols were named as S0, S0.005, S0.01, S0.02, S0.05 and S0.1 as the molar ratio of POTS to TEOS was 0, 0.005, 0.01, 0.02, 0.05 and 0.1, respectively. The sols were aged at 25 °C for 2 weeks before use. Finally, the silica sols were deposited on well-cleaned BK-7 substrates by dip coating. The central wavelength of the AR coating was adjusted to be about 500–600 nm by changing the withdraw rate during the coating process. The AR coatings were heat treated at 80 °C for 2 hours under an ambient atmosphere. The AR coatings were labeled as F0, F0.005, F0.01, F0.02, F0.05 and F0.1, respectively. More details about preparation of silica sols and AR coatings is provided in our previous studies.6,10,11
| TEOS/g | Isopropanol/g | Ammonia/g | POTS/g | |
|---|---|---|---|---|
| S0-9.2 | 15.6 | 130 | 9.2 | 0 |
| S0-4.6 | 15.6 | 130 | 4.6 | 0 |
| S0 | 15.6 | 130 | 2.3 | 0 |
| S0.005 | 15.6 | 130 | 2.3 | 0.23 |
| S0.01 | 15.6 | 130 | 2.3 | 0.45 |
| S0.02 | 15.6 | 130 | 2.3 | 0.90 |
| S0.05 | 15.6 | 130 | 2.3 | 2.17 |
| S0.1 | 15.6 | 130 | 2.3 | 4.15 |
Ethanol is the most common dispersant of silica sols in the preparation of AR coatings. At the beginning we also chose ethanol as the dispersant in the preparation of fluoro-containing ORMOSIL sols. However, POTS is difficult to dissolve in ethanol; moreover, POTS oil drops can be observed at the bottom of glass container. After ageing for several days, the insoluble POTS drops turned into a precipitate. This indicated that little POTS had been incorporated into the silica particles by co-condensation of TEOS and POTS. Therefore, a good solvent for POTS must be used as the new dispersant in the preparation of fluoro-containing ORMOSIL sols.
Isopropanol can dissolve POTS well and hence was used as the dispersant in this work. Therefore, in this work, a new formula was optimized for the preparation of a silica AR coating with isopropanol as the dispersant. To obtain a silica AR coating with nearly 100% transmittance, the particle size of the silica particles should be about 20 nm.3 The particle size of the silica particles from the Stöber method can be conveniently controlled by adjusting the ammonia content.13 Fig. 1(a) shows the particle size of silica sols with different ammonia contents. By decreasing the ammonia content from 9.2 g to 2.3 g, the particle size of the silica sols decreased from 254 nm to 15 nm. Thin films from S0, S0-4.6 and S0-9.2 were coated to test their refractive index; however, a thin film from S0-9.2 was difficult to realize. Therefore, only the refractive index of thin films from S0 and S0-4.6 were tested and shown in Fig. 2. As shown in Fig. 2, the refractive index of the thin films from S0 and S0-4.6 at 550 nm is about 1.22 and 1.15, respectively. This indicates that the thin film deposited by bigger particles possesses a lower refractive index.10 Moreover, the refractive index of F0 (thin film from S0) is equal to the square root of that of the substrate, and therefore it can be deduced that F0 would have nearly 100% transmittance. Therefore, the ammonia content was set as 2.3 g.
Fig. 1(b) shows the effect of the POTS content on particle size. As shown in Fig. 1(b), as the molar ratio of POTS to TEOS increased from 0 to 0.05, the particle size of the silica sols increased very slightly from 15 nm to 18 nm. However, the particle size was almost tripled to 43 nm as the molar ratio of POTS to TEOS increased to 0.1.
Fig. 3 shows the water contact angle images of the AR coatings with different molar ratios of POTS to TEOS. It is a very interesting phenomenon that the water contact angle of F0, which is fluoro-free, is 142°. It is well known that inorganic silica AR coatings from the sol–gel method is totally hydrophilic because the surface of the silica particles is covered by hydroxyl groups.6,11 In this work, we replaced ethanol with isopropanol because POTS cannot be well dissolved in ethanol. Isopropanol was intended to be used as the dispersant to dissolve POTS to obtain homogeneous fluoro-containing silica sols. However, the water contact angle result reveals that isopropanol might actively participate in some reactions and replace the –OH groups on surface of silica particles with –OCH(CH3)2. After the silica particles were covered by hydrophobic –OCH(CH3)2 groups, the silica AR coating was almost superhydrophobic. The presence of –OCH(CH3)2 groups in silica particles was demonstrated by FTIR as shown in Fig. 4. By increasing the molar ratio of POTS to TEOS from 0 to 0.1, the water contact angle remained almost unchanged. This is because the AR coatings were almost superhydrophobic without POTS, it is thus very hard to further improve the hydrophobicity of the AR coating.
The FTIR spectra of silica powders from silica sols with different POTS contents were recorded with a Bruker Tensor 27 using the KBr method in transmission mode. All silica were extracted exhaustively with isopropanol for 24 hours using the Soxhlet apparatus to remove the by-products from the self-condensation of POTS. With ethanol as the dispersant, the silica AR coating was hydrophilic.6,11 Between 2000 cm−1 and 600 cm−1, the hydrophilic silica had only three absorption bands at 1068 cm−1, 956 cm−1 and 802 cm−1, which are assigned to Si–O–Si and Si–OH.10,14 However, with isopropanol as the dispersant (S0 in Fig. 4), there were four additional adsorption bands at about 1470 cm−1, 1375 cm−1, 1386 cm−1 and 897 cm−1. Two absorption bands at 1375 cm−1 and 1386 cm−1 with same intensity were very characteristic absorption bands for the –CH(CH3)2 groups. This demonstrated that the –OCH(CH3)2 groups were covalently bonded to the silica particles. In the spectra of the fluoro-containing silica, further absorption bands assigned to C–F bonds were revealed at 1209 cm−1 and 721 cm−1.15 These absorption bands increased gradually as the molar ratio of POTS to TEOS increased. The most important peak appeared at 1145 cm−1.16 The absorption bands for C–F bonds confirmed that the perfluoroalkyl chains were clearly attached to the silica particles.
Transmittance is the most fundamental property for AR coatings. The transmittance of AR coatings used in high-powered laser systems should be higher than 99.5%. Fig. 5 (a) shows the transmittance spectra of AR coatings with different POTS contents. The transmittance of the AR coating was unchanged to be nearly 100% while the molar ratio of POTS to TEOS increases from 0 to 0.05. Silica AR coatings from the sol–gel Stöber method consist of a layer of silica particles, which stack randomly on the substrate. The refractive index of the dense silica thin film is about 1.46. With the appropriate particle size, the pores between silica particles lower the refractive index porous silica thin films to the square root of that of substrate, and hence the silica thin films would possess nearly 100% transmittance. As shown in Fig. 5(b), F0.05 is a porous thin film stacked by silica particles. As the molar ratio of POTS to TEOS increased to 0.1, the transmittance decreased to 98.2%. The particle size of S0.1 is three times as that of S0.05. The big particles result in a low refractive index.10 The refractive index of AR coating for NIF should be about 1.22 to give 100% transmittance. The deviation of the refractive index of F0.1 from the optimal value obviously decreased the transmittance. Moreover, the big particles also lead to light scattering on coating's surface.
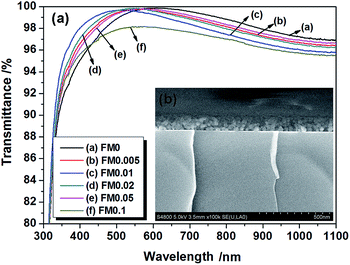 | ||
| Fig. 5 Transmittance spectra of AR coatings with different POTS contents (a) and cross sectional SEM image of F0.05 (b). | ||
The environmental resistance of AR coatings in high-powered laser systems is very important. Hydrophilic silica AR coatings have poor environmental resistance. As shown in Fig. 6, by exposure to a humid environment (about 95% humidity) for 2 months, the transmittance of a hydrophilic silica AR coating, which was prepared from silica sol with ethanol as the dispersant, decreases from 99.9% to 98.5%; however, the transmittance of F0 and F0.05 decreased only from 99.9% to 99.5% and 99.8% to 99.6%, respectively. This indicates that both the fluoro-free and fluoro-containing silica AR coatings possess good environmental resistance, which is an unexpected result. At the beginning, we believed that F0 would have poor environmental resistance and the environmental resistance of AR coatings would be gradually improved with increasing the POTS content. It is not clear whether POTS makes a contribution to the environmental resistance.
 | ||
| Fig. 6 Environment-resistance of F0 and F0.05 AR coatings to hydrophilic and hydrophobic pollutants. | ||
In conclusion, a sol–gel process was proposed to prepare environment-resistant fluoro-containing AR coatings by using TEOS and POTS as co-precursors and isopropanol as the dispersant. FTIR spectra results demonstrated that perfluoroalkyl chains were covalently linked to the silica particles. Although it is not clear whether POTS makes contribution to the environmental resistance, this work is still valuable. On the basis of this work, further research on the amphiphobic property of fluoro-containing AR coatings will be carried out due to the very low surface energy of perfluoroalkyl chains. The amphiphobic AR coatings would have environmental resistance to both hydrophobic and hydrophilic pollutants in high-powered laser systems.
Acknowledgements
The authors gratefully acknowledge the support from Natural Science Foundation of China (31770535).Notes and references
- E. I. Moses, J. H. Campbell, C. J. Stolz and C. R. Wuest, Proc. SPIE, 2003, 5001, 1–15 CrossRef PubMed.
- T. J. Jacobsson and T. Edvinsson, RSC Adv., 2012, 2, 10298–10305 RSC.
- P. K. Whitman, S. C. Frieders, J. Fair, I. M. Thomas, R. Aboud, C. B. Thorsness and A. K. Burnham, ICF Quarterly Report, 1999, 9, pp. 163–176 Search PubMed.
- I. M. Thomas, Proc. SPIE, 1994, 2114, 232–243 CrossRef CAS PubMed.
- I. M. Thomas, A. K. Burnham, J. R. Ertel and S. C. Frieders, Proc. SPIE, 1999, 3492, 220 CrossRef CAS PubMed.
- X. X. Zhang, S. Cai, D. You, L. H. Yan, H. B. Lv, X. D. Yuan and B. Jiang, Adv. Funct. Mater., 2013, 23, 4361–4365 CrossRef CAS.
- L. Xu, L. Gao and J. He, RSC Adv., 2012, 2, 12764–12769 RSC.
- X. Zhang, F. Zheng, L. Ye, P. Xiong, L. Yan, W. Yang and B. Jiang, RSC Adv., 2014, 4, 9838–9841 RSC.
- Y. Xu, L. Zhang, Y. H. Sun, Z. X. Huang, X. D. Jiang, X. F. Wei, Z. H. Li, B. Z. Dong and Z. H. Wu, J. Opt. Soc. Am. B, 2005, 22, 905–912 CrossRef CAS.
- X. Zhang, B. Xia, H. Ye, Y. Zhang, B. Xiao, L. Yan, H. Lv and B. Jiang, J. Mater. Chem., 2012, 22, 13132–13140 RSC.
- X. Zhang, C. Cao, B. Xiao, L. Yan, Q. Zhang and B. Jiang, J. Sol-Gel Sci. Technol., 2010, 53, 79–84 CrossRef CAS PubMed.
- G. Zhou, J. He, L. Gao, T. Ren and T. Li, RSC Adv., 2013, 3, 21789–21796 RSC.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- M. J. Mosquera, D. M. Santos, A. Montes and L. Valdez-Castro, Langmuir, 2008, 24, 2772–2778 CrossRef CAS PubMed.
- T. Monde, H. Fukube, F. Nemoto, T. Yoko and T. Konakahara, J. Non-Cryst. Solids, 1999, 246, 54–64 CrossRef CAS.
- R. Tian, O. Seitz, M. Li, W. Hu, Y. J. Chabal and J. Gao, Langmuir, 2010, 26, 4563–4566 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |




