Simultaneous deposition of cerium oxide and gold nanostructures-characterization and analytical properties toward glucose electro-oxidation and sensing†
Abstract
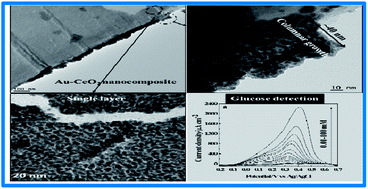
Here, we present for the first time an Au–CeO2 nanocomposite synthesized in the presence of a mild pressure of oxygen (1.33 Pa) by the cross-beam laser deposition technique. The deposit was grown on a conductive (current collector) substrate composed of a porous network of carbon microfibers. The structure and morphology of the deposit were characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). XPS analysis demonstrated that the interaction of Au with CeO2 during deposition induced charge redistribution in the ceria nanoparticles (Ce3+,4+), resulting in positively charged Au species (Au3+). TEM analysis revealed that the basal planes of the carbon fibers are coated with a monolayer of interconnected nanoparticles, whereas their outer walls are covered with an ultra-thin film (∼40 nm thickness) formed of nanoparticles gathering into columns. The direct electrocatalytic oxidation of glucose in a neutral medium, such as phosphate buffered saline (pH = 7.2) solution, has been investigated with cyclic voltammetry, chronoamperometry and square-wave voltammetry. Sensitivity as high as 57.5 μA cm−2 mM−1 up to 10 mM of glucose and a low detection limit of 10 μM were obtained with square-wave voltammetry. Such electrocatalytic activity is because of the presence of cationic gold (electronic effect) and the size reduction of gold particles (size effect). This interesting analytical performance in addition to the low loading of gold (100 μg cm−2) makes the laser fabricated Au–CeO2 nanocomposite electrode potentially promising for glucose fuel cells, assessment of glucose in biological fluids, as well as in the analysis of food and beverages.

 Please wait while we load your content...
Please wait while we load your content...