Oil gels with a chemically cross-linked copolymer of a trimethylene carbonate derivative and l-lactide: preparation and stereocomplex formation within gels†
Abstract
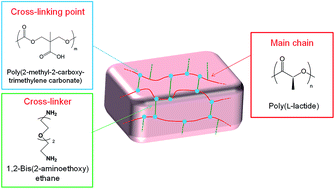
Oil gels made of low-toxic components were prepared using chemically cross-linked copolymer, which was composed of a poly(trimethylene carbonate) derivative and poly(L-lactide). The poly(L-lactide) moiety in the gels could form stereocomplexes with poly(D-lactide).

 Please wait while we load your content...
Please wait while we load your content...