Catalytic combustion of low-concentration coal bed methane over CuO/γ-Al2O3 catalyst: effect of SO2
Z. Yang*a,
J. Liua,
L. Zhanga,
S. Zhenga,
M. Guoab and
Y. Yana
aKey Laboratory of Low-grade Energy Utilization Technologies and Systems, Chongqing University, Ministry of Education of PRC, Shapingba District, Chongqing, 400030, China. E-mail: zqyang@cqu.edu.cn; Fax: +86-23-65111832
bCollege of Mechanical and Power Engineering, Chongqing University of Science and Technology, Shapingba District, Chongqing 401331, China
First published on 11th August 2014
Abstract
The purpose of this work is to report the results of an experimental study devoted to investigate the effects of SO2 in feed gas on the catalytic combustion of low-concentration coal bed methane (1 vol%) over CuO/γ-Al2O3 catalyst with 10 wt% Cu prepared by incipient wetness impregnation in a fix-bed reactor. The study deals with researching a regular low-concentration coal bed methane combustion with lean SO2, which is vital in utilizing low-concentration coal bed methane and in eliminating greenhouse emissions. The concentration of SO2 varied from 0 ppm to 200 ppm, and the accumulation of sulfur on the catalyst surface was examined by X-ray fluorescence (XRF) and Scanning electron microscopy (SEM). The reaction temperature was in the range of 450–700 °C controlled by an electric heater, and at two key reaction temperatures (550 °C and 650 °C), the effect of altering SO2 concentration on low-concentration coal bed methane combustion was studied experimentally. A sulfur poisoning mechanism of CuO/γ-Al2O3 catalyst was proposed. The results showed that the presence of SO2 in feed gas led to the phenomena of sulfur poisoning over CuO/γ-Al2O3 catalyst, and the effect of sulfur poisoning aggravated with increased SO2 concentration. Meanwhile, it had significant influence when temperature was below 575 °C due to the faster decomposition of sulfates at higher temperatures. A high reaction temperature promoted the decomposition of sulfates produced simultaneously, which provided more activating sites on the catalyst for methane catalytic combustion. Sulfates (CuSO4 and Al2(SO4)3) were detected by Fourier transform-infrared spectroscopy (FT-IR) and X-ray diffraction (XRD), resulting in both the reduction of activating sites and specific surface area of the catalyst. The absorption of methane molecules on activated sites was hindered and the catalyst activity decreased.
Introduction
Coal bed methane (CBM) is a form of natural gas extracted from coal beds. The major combustible component of CBM is methane, which varies from 0.1–95 vol% according to mining conditions.1 Su et al.2 classified CBM emission in three streams based on the description of methane released prior to, during, and after mining operations. Mine ventilation air with methane concentration of 0.1–1 vol% is the CBM released during coal mining. It is difficult to utilize with conventional technologies because of its lean methane concentration. Most of the mine ventilation air is emitted to the atmosphere without any treatment. Methane emitted from coalmine contributes 17% to the total anthropogenic greenhouse emissions. In China, the total amount of methane emitted to the atmosphere is almost 20 × 109 m3 every year, and the value is increasing with the growth of coal production.3 Note that methane itself is a greenhouse gas with global warming effect 21–23 times greater than that of carbon dioxide.1Catalytic combustion is a clean and green technology for burning low-concentration CBM because of its potential to produce heat and energy at a relatively low temperature with very little production of CO, hydrocarbons and NOx than conventional flame combustion. It is considered to be one of the most effective ways to utilize low concentration CBM.4–7 The catalytic combustion of methane has been of wide concern in recent years. Noble metal catalysts are generally used in methane combustion experiments for its advantage of excellent combustion activity. Oh and coworkers investigated methane combustion over Al2O3-supported catalysts and reported that the activity for methane combustion increased in the following order: Pt < Rh < Pd.8 Furthermore, some studies reported that the activity and durability of Pd/γ-Al2O3 was improved by the addition of Pt.9–13 However, the high cost of noble metal catalysts hinders their large-scale industrial application. Copper-based oxide catalyst which has broad application prospect for its low cost, is one of the highest activity transition metal oxide catalysts for methane combustion.14–16 Rui et al. studied the influence of Cu-based catalyst on the combustion characteristics of CO and methane and reported the catalytic activity of CuO on a unit surface similar to that of noble metal, Cu-based catalyst with different carriers also can make methane combustion and conversion complete at temperatures between 550 and 670 °C.14,15 Zhang et al. studied the combustion characteristics of ultra-low concentration methane in a fluidized bed with CuO/γ-Al2O3 catalyst particles. It was found that complete conversion could be achieved at 600 °C.16 The complete combustion mechanism of methane can be represented simply by the following eqn (1):
| CH4 + 2O2 → CO2 + 2H2O, ΔH(298) = −802.7 kJ mol−1 | (1) |
It is important to note that there is a small amount of sulfur dioxide in the low-concentration CBM. Although the sulfur dioxide concentration is less than 200 ppm under most conditions, it has a significant influence on the catalytic combustion reaction. Catalysts are poisoned with the presence of SO2, and methane conversion reduces obviously during the catalytic combustion of the low concentration CBM. The poisoning effect of sulfur on Pd/γ-Al2O3 by SO2 has been studied widely in the last few decades.17–19 It was found that the deactivation of the catalyst was accelerated with the increasing SO2 concentration, and the formation of surface PdSO4 was the major cause of deactivation. For low concentration CBM combustion over CuO/γ-Al2O3, the effect of SO2 on catalytic combustion characteristic should be further investigated. In this paper, the CuO/γ-Al2O3 catalyst was prepared with incipient wetness impregnation method, the effects of SO2 on the low-concentration CBM catalytic combustion over CuO/γ-Al2O3 were investigated, the micro-structures of the catalysts before and after the reactions were analyzed, and the mechanism of reaction is discussed.
Experimental
Catalyst preparation
CuO/γ-Al2O3 catalyst was prepared by incipient wetness impregnation with γ-Al2O3 particles (9–16 mesh) as the carrier and Cu(NO3)2·6H2O (Aldrich 99.99%) as the solution at room temperature. After impregnation for 10 h, the catalyst was dried in an oven at 110 °C for 12 h, and then calcined in a muffle furnace at 600 °C for 4 h (called fresh catalyst). X-ray fluorescence spectrometer (XRF-1800, Japan) was used to analyze elemental contents (Al, Cu, O, S) in the catalysts, and the data are shown in Table 1.| The station of catalyst | The main atom ratios (wt%) | |||
|---|---|---|---|---|
| Al | O | Cu | S | |
| Fresh catalyst | 41.55 | 38.77 | 10.82 | — |
| Catalyst after reaction without SO2 | 41.06 | 38.93 | 10.77 | — |
| Catalyst after reaction with 200 ppm SO2 | 41.73 | 39.67 | 10.28 | 0.344 |
Experimental setup
The catalytic combustion tests of low-concentration CBM with CuO/γ-Al2O3 as the catalyst were carried out in a fixed bed reactor at atmospheric pressure. A sketch of experimental system is shown in Fig. 1. The reactor was a ceramic tube (1.2 m long, 10 mm I.D.) placed in a horizontal tube furnace. 1 g catalysts were loaded in the middle of the reactor and fixed by two quartz wool plugs. Low-concentration CBM with its methane concentration of 1 vol% and SO2 concentration of 0–200 ppm was simulated with methane (99.99%, Ruixin, Inc.), SO2 (10%, Ruixin, Inc.) and air as the balance gas. The total flow rate of low-concentration CBM was 200 mL min−1 for all the experiments. The reaction temperature varied from 475 °C to 700 °C and was increased by 25 °C every 15 min by an electric heater equipped with temperature control. Gas samples were withdrawn at the outlet of the reactor and measured by a gas chromatograph (Techcomp-GC7900).Methane conversion is defined as the following eqn (2):
 | (2) |
The production of sulfate ion on CuO and γ-Al2O3 was investigated by an FT-IR infrared spectrometer (NicoletiN10, America). XRD analysis was performed on fresh and aged catalysts samples with an X-ray diffractometer (D/Max-3C, Japan). The analysis of BET specific surface area and porosity were carried out with an analyzer (ASAP2020V3.04H, America) by measuring absorption–desorption isothermal lines of nitrogen in the samples. The surface structures of the samples were observed by an Scanning electron microscope (SEM, VEGA3 SBH, Czechoslovakia). Thermal gravimetric analysis was conducted with a thermogravimetric analyzer (STA-409-PC, Germany) to achieve the rate of weight loss of the catalyst samples in the nitrogen feed stream.
Results and discussions
Effect of SO2 on methane conversion
Fig. 2 shows the effects of SO2 concentration (0–200 ppm) on methane conversions over the CuO/γ-Al2O3 catalyst at temperature 475–700 °C. It is clearly observed that methane conversion increases with increasing reaction temperature, and achieves about 100% at 700 °C without SO2. Methane conversion decreases with increasing SO2 concentration at the same reaction temperature. It is suggested that the presence of SO2 in the feed gases has a significant negative effect on the activity of CuO/γ-Al2O3. Compared with the reaction in the absence of SO2, T10 increased from 483 °C to 494 °C, and T90 also increased from 614 °C to 650 °C in the presence of 50 ppm SO2. Then, as SO2 concentration in the feed gases increased to 200 ppm, T10 and T90 increase to 54 °C and 86 °C, respectively. This indicates that the addition of SO2 decreases the activity of CuO/γ-Al2O3 catalyst. This is a plausible reason that SO2 reacts with CuO to form sulfate on the catalyst surface and occupies the surface, which reduces the number of activated sites for methane absorption. As SO2 concentration is increased, much more sulfate occupies active sites on the catalyst. Therefore, the catalyst activity decreases with increasing SO2 concentration. | ||
| Fig. 2 Methane conversion over CuO/γ-Al2O3 catalyst with SO2 (CH4: 1 vol%; catalysts loading: 1 g; feed volume flow rate: 200 mL min−1). | ||
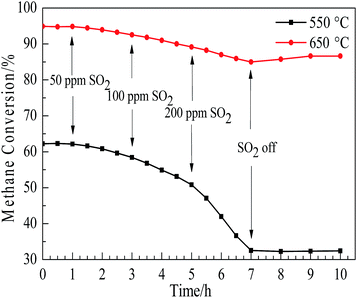
Fig. 3 shows the activity of the CuO/γ-Al2O3 catalysts with altering SO2 concentration between 550 °C and 650 °C. The flow rate of SO2 was controlled by a mass flow meter. In the first hour, SO2 concentration was at a level of 0 ppm, at one hour, SO2 concentration increased from 0 ppm to 50 ppm, which was held for an hour and then the SO2 concentration was doubled every two hours until reaching seven hours when the SO2 concentration was set to 0 ppm. As the SO2 concentration increased, a clear decline in methane conversion was observed at 550 °C and 650 °C at seven hours. The decline in amplitude is larger at 550 °C than that at 650 °C in the first seven hours. It is a reasonable elucidation that the sulfates formed at low temperature inhibit methane absorption on the catalysts activated sites, and the decomposition of sulfates at 650 °C occurs faster than that at 550 °C, leading to the higher net formed rate of sulfates at 550 °C. With the SO2 removed from the feed gas after seven hours, methane conversion at 650 °C increases slightly, and methane conversion at 550 °C remained stable. This is because after the removal of SO2 at 650 °C, the former accumulative sulfates decompose, which provide more activated sites for methane catalytic combustion. In the next section, the thermal gravimetric study also shows that the sulfate decomposition occurs when the temperature exceeds 575 °C.
Effects of SO2 on CuO/γ-Al2O3 catalyst
Table 1 shows the elemental content (Al, O, Cu, S) of the CuO/γ-Al2O3 catalyst in the absence and presence of 200 ppm SO2 in feed gases. The results of XRF analysis prove that sulfur accumulates in catalysts after reaction with 200 ppm SO2 in feed gases, and a sulfur content of 0.344 wt% was determined. In the absence of SO2, oxygen concentration is 38.77% (before reaction) and close to 38.93% (after reaction), which has a non-negligible increase of 0.9% in the presence of 200 ppm SO2. It is reasonable to conclude that the accumulation of sulfur in the form of S6+ carries with it a certain amount of oxygen.20 CuSO4 and Al2(SO4)3 are the probable sulfates.The abovementioned discussions speculate the existence of sulfates. FT-IR analysis was carried out on the aged catalysts. An obvious sulfate absorption (1126 cm−1) peak can be seen with 200 ppm SO2, as shown in Fig. 4. According to Ordonez and Hurtado's study,21 high temperatures (>700 °C) promote the formation of aluminium sulfate. Therefore, sulfates ought to consist of massive amounts of copper sulfate species and a slight amounts of aluminium sulfate species.
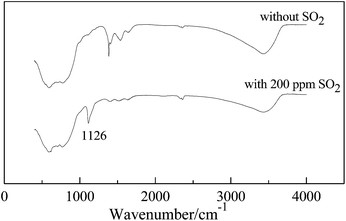 | ||
| Fig. 4 FT-IR image of CuO/γ-Al2O3 catalyst after reaction (CH4: 1 vol%; catalyst loading: 1 g; feed volume flow rate: 200 mL min−1). | ||
Fig. 5 shows XRD spectra of fresh and aged catalysts. Obvious diffraction peaks of CuO and Al2O3 can be observed on three catalysts. However, the XRD images of poisoned catalysts demonstrate diffraction peaks, which represent sulfate (CuSO4 and Al2(SO4)3). It is confirmed that CuSO4 and Al2(SO4)3 are formed in the poisoned catalysts.
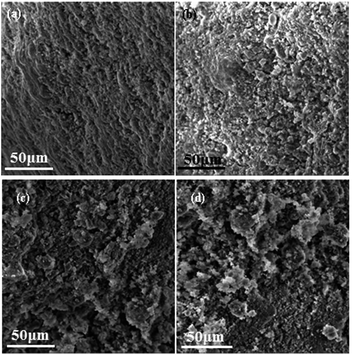
Specific surface areas of fresh and aged catalysts are shown in Table 2. Compared with the fresh catalyst, the specific surface area of the catalyst aged without SO2 has a negligible change, which varies from 94.2 m2 g−1 to 92.3 m2 g−1. However, the specific surface areas of the aged catalysts with 100 and 200 ppm SO2 present in feed gas reduce to 85.8 m2 g−1 and 80.4 m2 g−1, respectively. It is justifiable to conclude that the specific surface area of aged catalysts decreases clearly when SO2 is present in the feed gas, and the variation of specific surface area increases with increasingSO2 concentration. A plausible reason of this is that sulfate formed on the catalysts surface is in the form and attaches to the catalyst surface to form a hard shell, which can also be seen in Fig. 6. This leads to the reduction of specific surface area of the poisoned catalysts by blocking the methane molecule from diffusing into the activated sites, resulting in a decrease of methane conversion which is observed in Fig. 2 and 3.
| Before reaction | After reaction | ||
|---|---|---|---|
| CH4: 1 vol% SO2: 0 ppm | CH4: 1 vol% SO2: 100 ppm | CH4: 1 vol% SO2: 200 ppm | |
| 94.2 m2 g−1 | 92.3 m2 g−1 | 85.8 m2 g−1 | 80.4 m2 g−1 |
The weight loss curves of catalysts in a N2 atmosphere with a temperature ramp of 20 °C min−1 are compared in Fig. 7. When temperature is below 575 °C, the two curves show the same trend, and the weight loss of SO2 poisoned catalyst is slightly larger than that of the normal catalyst. This is mainly because SO2 absorbed on the poisoned catalysts is released under the condition of heating. When the temperature exceeds 575 °C, the weight loss curve of normal catalyst maintains stable, while the other develops a remarkable mass loss, suggesting that the decomposition of sulfate mentioned above occurred above 575 °C. As we know, the decomposition temperatures of CuSO4 and Al2(SO4)3 are about 570 °C and 770 °C, respectively, which will explain the degree of weight loss of the poisoned catalyst above 575 °C (the decomposition of CuSO4) and above 770 °C (decomposition temperature of CuSO4 and Al2(SO4)3). When the temperature is over 1000 °C, the TG curve of poisoned catalysts stay at a level that indicates the sulfate undergoes a saturated decomposition. Davis et al.22 launched a mass spectrometry test and discovered that the main content of production after the decomposition of poisoned Pd-based catalysts was SO3, however, which is converted into O2 and SO2 at high temperature. Therefore, the TG curve showing a drop of poisoned catalyst mass is explained by the escape of O2 and SO2.
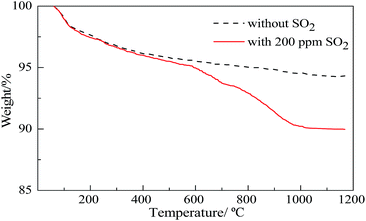 | ||
| Fig. 7 Weight loss curves of normal and poisoned catalyst (normal catalyst: aged without SO2; poisoned catalyst: aged with 200 ppm SO2). | ||
The mechanism of SO2 on CuO/γ-Al2O3 catalyst deactivation
By combining the findings stated above, the presence of SO2 in the feed gases is the reason that CuO/γ-Al2O3 catalyst undergoes the phenomena of sulfur poisoning. According to Ordonez and Hurtado's study on noble metal catalysts,21 the sulfur poisoning of Pd catalyst proceeds through two consecutive steps: adsorption of SO2 and reaction of the adsorbed SO2 to form Pd sulfites or sulfates. Meanwhile, Pd sulfites or sulfates react with Al2O3 to form Al sulfites or sulfates. Therefore, the following global mechanism of SO2deactivation of CuO/γ-Al2O3is proposed:| SO2 + CuO → CuO·SO2 | (3) |
| 2CuO·SO2 + O2 → 2CuO·SO3 | (4) |
| CuO·SO3 → CuSO4 | (5) |
| CuO·SO3 + Al2O3 → Al2O3·SO3 + CuO | (6) |
| Al2O3·SO3 + 2SO2 + O2 → Al2(SO4)3 | (7) |
SO2 absorbs to the activated sites on CuO as the reaction (3), and being oxidized to SO3 in the form of adsorption state according to the reaction (4), and then CuSO4 is produced by the reaction (5) between SO3 and CuO. At the same time, adsorption state SO3 moves to near dissociating sites of Al, and reaction (3)–(6) are followed with reaction (7) producing Al2(SO4)3. Because of the formation of sulfate, some activated sites of CuO are blocked, leading to catalyst deactivation and decrease in methane conversion.
Conclusions
The effects of SO2 on the catalytic combustion of low-concentration CBM over a CuO/γ-Al2O3 catalyst was investigated experimentally in a fix-bed reactor, the conclusions are as follows:(1) The presence of SO2 in the feed gas leads to the phenomena of sulfur poisoning over the CuO/γ-Al2O3 catalyst, and this leads to a decline in catalyst activity. Methane conversion decreased with increasing SO2 concentration.
(2) SO2 in the feed gas has more significant influence on methane conversion over CuO/γ-Al2O3 catalyst when temperature is below 575 °C. High reaction temperatures promote the decomposition of sulfate products simultaneously, which provides more activating sites for methane catalytic combustion.
(3) The mechanism of SO2 on CuO/γ-Al2O3 catalyst deactivation was proposed, the reason of sulfur poisoning of CuO/γ-Al2O3 catalyst is that sulfates (CuSO4 and Al2(SO4)3) are formed on the catalyst surface. Activated sites are then occupied and the specific surface area of the catalyst is reduced. The absorption of methane molecules on activated sites is hindered and then catalyst activity decreases.
Acknowledgements
The authors would like to thank the financial support from Natural Science Foundation of China with Project no. 51206200, and the Fundamental Research Funds for the Central Universities with Project no. CDJZR12140031, and visiting Scholar Foundation of Key Lab. of Low-grade Energy Utilization Technologies and System, MOE of China in Chongqing University (LLEUTS-201301).Notes and references
- Z. Yang, J. R. Grace, C. J. Lim and L. Zhang, Energy Fuels, 2011, 25(3), 975–980 CrossRef CAS.
- S. Su, A. Beath, H. Guo and C. Mallett, Prog. Energy Combust. Sci., 2005, 31(2), 123–170 CrossRef CAS PubMed.
- G. Groppi, C. Cristiani, L. Lietti, C. Ramella, M. Valentini and P. Forzatti, Catal. Today, 1999, 50(2), 399–412 CrossRef CAS.
- Y. Wang, Y. Liu, Q. Cao, C. Wang and D. Che, Energy Fuels, 2011, 25(8), 3437–3445 CrossRef CAS.
- J. Okal and M. Zawadzki, Appl. Catal., A, 2013, 453, 349–357 CrossRef CAS PubMed.
- K. Sekizawa, H. Widjaja, S. Maeda, Y. Ozawa and K. Eguchi, Catal. Today, 2000, 59(1), 69–74 CrossRef CAS.
- T. V. Choudhary, S. Banerjee and V. R. Choudhary, Appl. Catal., A, 2002, 234(1–2), 1–23 CrossRef CAS.
- K. R. Chong, W. R. Min and S. R. In, Catal. Today, 1999, 47(1–4), 141–147 Search PubMed.
- H. Ohtsuka, Catal. Lett., 2011, 141(3), 413–419 CrossRef CAS.
- P. O. Thevenin, A. Alcalde and L. J. Pettersson, J. Catal., 2003, 215(1), 78–86 CrossRef CAS.
- S. Colussi, F. Arosio and T. Montanari, Catal. Today, 2010, 155(1), 59–65 CrossRef CAS PubMed.
- S. Ordóñez, P. Hurtado and H. Sastre, Appl. Catal., A, 2004, 259(1), 41–48 CrossRef PubMed.
- X. Zi, L. Liu and B. Xue, Catal. Today, 2011, 175(1), 223–230 CrossRef CAS PubMed.
- Z. Rui, Y. Huang and Y. Zheng, J. Mol. Catal. A: Chem., 2013, 372, 128–136 CrossRef CAS PubMed.
- Y. Li, Y. Guo and B. Xue, Fuel Process. Technol., 2009, 90(5), 652–656 CrossRef CAS PubMed.
- L. Zhang, J. Zhang and Z. Yang, J. Fuel Chem. Technol., 2012, 40(7), 886–891 CAS.
- T. C. Yu and H. Shaw, Appl. Catal., B, 1998, 18(1), 105–114 CrossRef CAS.
- L. S. Escandón, S. Ordonez and A. Vega, J. Hazard. Mater., 2008, 153(1), 742–750 CrossRef PubMed.
- S. Ordóñez, P. Hurtado and H. Sastre, Appl. Catal., A, 2004, 259(1), 41–48 CrossRef PubMed.
- J. R. Esteban and H. S. Kirk, Appl. Surf. Sci., 2005, 246(3), 262–270 Search PubMed.
- S. Ordonez, P. Hurtado and F. V. Díez, Catal. Lett., 2005, 100(1–2), 27–34 CrossRef CAS PubMed.
- B. H. Davis, R. A. Keogh and R. Srinivasan, Catal. Today, 1994, 20(2), 219–256 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |