Synthesis of micrometer-sized hierarchical rutile TiO2 flowers and their application in dye sensitized solar cells†
Abstract
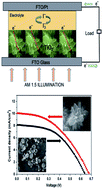
Cactus-like hierarchical rutile TiO2 flowers and three dimensional (3D) highly branched rutile TiO2 nanorods with sizes measuring up to 5 microns were synthesized on conductive substrates by a facile hydrothermal route without the presence of a surfactant or template. These samples with different morphologies and microstructures were studied by X-ray powder diffraction (XRD), field emission-scanning electron microscopy (FESEM) and high resolution transmission electron microscopy (HRTEM). We also studied the photovoltaic performances of these samples by using them as photoanodes in dye-sensitized solar cells (DSSCs). The highly branched TiO2 nanorod based photoanode in DSSCs showed a power conversion efficiency of 3.07% which was significantly higher than that of the cactus TiO2 flower based (2.66%) photoanode. The electrochemical impedance spectroscopy (EIS) analysis of the interfacial charge transfer kinetics in these photoanodes in DSSCs showed higher recombination resistance (R2) and longer electron lifetime in highly branched nanorods. The enhancement of the efficiency of the highly branched TiO2 nanorod photoanode based DSSC compared to that of cactus TiO2 flower DSSC is mainly attributed to the superior light scattering capability, fast electron transfer and longer electron lifetime with suppressed recombination.

 Please wait while we load your content...
Please wait while we load your content...