In vitro DNA binding, molecular docking and antimicrobial studies on a newly synthesized poly(o-toluidine)–titanium dioxide nanocomposite†
Abstract
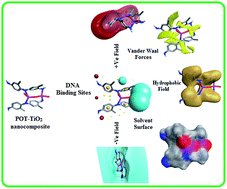
A poly(o-toluidine)–titanium dioxide (POT–TiO2) nanocomposite has been synthesized for the first time by in situ chemical oxidative polymerization of o-toluidine (OT) in the presence of titanium dioxide (TiO2) nanoparticles. FTIR, SEM, TEM, XRD, TGA and DTA were used to characterize the POT–TiO2 nanocomposite. The characterization results confirmed that there is a strong interaction between POT and TiO2 nanoparticles and the nanocomposite showed higher thermal stability than pristine POT. The in vitro DNA binding studies of POT and POT–TiO2 nanocomposite with ct-DNA in physiological buffer (pH-7.4) were investigated using spectrophotometry and circular dichroism. The absorption spectra of the POT and POT–TiO2 nanocomposite with DNA showed a hypochromic effect. The four types of 3D molecular field descriptors or field points as extrema of electrostatic, steric, and hydrophobic fields are described. These field points are used to define the properties necessary for a molecule to bind in a characteristic way into a specified active site. A molecular docking simulation was used to predict the modes of interactions of the drugs (POT and POT–TiO2) with DNA. The molecular docking results indicated that the modes of interactions between the two (POT and POT–TiO2) and DNA helix can be considered as groove binding. Moreover, the comparative antimicrobial activities of POT and its POT–TiO2 nanocomposite were tested and were found to exhibit antibacterial activity against Gram positive as well as Gram negative bacterial strains at micromolar concentration.

 Please wait while we load your content...
Please wait while we load your content...