Simple hydrothermal preparation of α-, β-, and γ-MnO2 and phase sensitivity in catalytic ozonation
Abstract
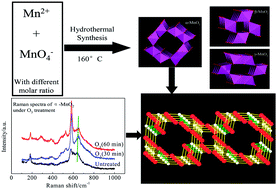
The investigation of the roles of bonds and phases in catalysis is of great significance for designing novel catalysts and proposing mechanisms. Hindered by the lack of comparable catalyst samples, the influence of bonds and phases on the instantaneous reaction between ozone and MnO2 is still unknown. In this paper, α-, β-, and γ-MnO2 were prepared through a uniform hydrothermal method, and the phase sensitivity of the manganese dioxide catalysts in the ozonation process was investigated. Raman spectra indicated that bands transformed to a larger range in the window of 550–700 cm−1, which is considered to be the fingerprint region of manganese dioxides. During catalytic ozonation, ozone reacted with particular Mn–O bonds to generate more powerful oxygen species, leading to better oxidation efficiency compared to single ozonation. The active bonds, which are favourable to the activation of ozone, include bonds belonging to the pyrolusite type structure of γ-MnO2, corresponding to the [MnO6] octahedral frameworks of β-MnO2 and perpendicular to the direction of the [MnO6] octahedral double chains of α-MnO2. Thermogravimetric analysis confirmed that both active surface oxygen and lattice oxygen were responsible for the catalytic activity of α-MnO2, while lattice oxygen and the bonded manganese of MnO2 contributed to the catalytic activity of β- and γ-MnO2.

 Please wait while we load your content...
Please wait while we load your content...