Proton-conducting polymer electrolytes and their applications in solid supercapacitors: a review
Abstract
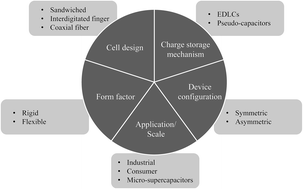
Research on solid supercapacitors over the last few years has aimed to provide high performing and safely operating energy storage solutions for the fast growing application areas of consumer and micro-electronics, providing printable, flexible and wearable devices. Most of the reported research has leveraged proton conducting polymer electrolytes for electrochemical double layer capacitors and pseudo-capacitors. In this paper, we provide an overview of the state-of-the-art solid supercapacitors enabled by proton-conducting polymer electrolytes. After a short overview of the types and configurations of solid supercapacitors, this review introduces proton-conducting polymers electrolytes and the mechanisms of proton conduction in a polymer matrix. Based on their chemistry, synthesizing method, and the nature of proton conduction, proton-conducting polymer electrolytes and the resultant supercapacitors are discussed in two categories: polymeric proton-conducting electrolytes and inorganic/polymer proton-conducting electrolytes. The performance and the technology gaps of the solid supercapacitors enabled by the presented polymer electrolytes are reviewed and compared. The review concludes with an outlook of future advancements required and the key research directions to achieving these.
- This article is part of the themed collection: Materials for Energy storage

 Please wait while we load your content...
Please wait while we load your content...