Metal–organic framework MIL-100(Fe) for artificial kidney application†
Cheng-Xiong Yang*,
Chang Liu,
Yi-Meng Cao and
Xiu-Ping Yan*
State Key Laboratory of Medicinal Chemical Biology (Nankai University), Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), and Research Center for Analytical Sciences, College of Chemistry, Nankai University, Tianjin 300071, China. E-mail: xpyan@nankai.edu.cn; Fax: +86 22 23506075
First published on 22nd August 2014
Abstract
Metal–organic framework MIL-100(Fe) was explored as a novel sorbent for artificial kidney application to remove a typical uremic toxin creatinine with high adsorption capacity and excellent reusability.
Renal failure is a syndrome where the kidneys fail to fulfill their blood purification function and leads to the accumulation of uremic toxins normally eliminated by healthy kidneys.1 Creatinine is one of the most important uremic toxins in the clinical analytic domain as it is used as a probe molecule to evidence renal failure. The accumulation of creatinine in the blood not only causes a series of toxic symptoms, but also reduces the kidney function and accelerates the renal decline.2 To reduce the pain of the patients from renal failure, it is very important to remove or eliminate the creatinine in the blood. At present, creatinine is commonly removed by hemodialysis in clinical treatment. However, creatinine cannot be effectively removed by a single hemodialysis process. To increase the epuration capacity of dialysis system, it is also possible to remove creatinine by adsorption onto solid materials.3 Therefore, exploring novel sorbents for creatinine adsorption have received great attention recently.4–11 To date, porous materials like activate carbon,4 zeolites,5–7 carbon nanotubes,8 and molecularly imprinted polymers9–11 for artificial kidney application of creatinine adsorption have been investigated.
Metal–organic frameworks (MOFs) are a novel class of porous materials constructed from metal ion or cluster nodes and organic linkers.12,13 The large surface area, diverse structures and pore topologies, accessible cages and tunnels make MOFs attractive for adsorptive application.14–19,24 MOFs with multiple functions and rigid pores may also be potential in artificial kidney application for the adsorption and removal of uremic toxins, such application, however, has never been reported so far.
Herein, we show a novel application of MOF MIL-100(Fe) as artificial kidney for the effective removal of a typical uremic toxin creatinine. MIL-100(Fe) is a crystalline three-dimensional iron(III) trimesate with mesoporous cages (25 and 29 Å), accessible windows (5.6 and 8.6 Å), large surface area, excellent chemical and solvent stability.20 MIL-100(Fe) was chosen as the sorbent because it is non-toxic,21 stable in physiological conditions22 and have the unique nano-sized channels to allow only the access of low molecular weight molecules.23 In addition, the large surface area also makes MIL-100(Fe) promising for creatinine adsorption.16,20
In this work, adsorption kinetics and thermodynamics for creatinine on MIL-100(Fe) were investigated in detail. MIL-100(Fe) is a highly potential sorbent for creatinine removal not only for its high adsorption capacity, but also for its easy desorption and excellent reusability.
MIL-100(Fe) was synthesized according to Horcajada et al.20 The structure of MIL-100(Fe) was confirmed by X-ray diffraction (XRD), thermal gravimetric analysis (TGA), scanning electron microscopy (SEM), and N2 adsorption (Fig. S1, ESI†). The synthesized MIL-100(Fe) gave a Brunauer–Emmett–Teller (BET) surface area of 1598 m2 g−1.
The time-dependent adsorption of creatinine on MIL-100(Fe) was investigated at three initial concentrations of creatinine solution (50, 100 and 200 mg L−1) at 37 °C. The selected concentrations were closed to the mean and the maximum creatinine concentration in the blood of patients with renal failure.6 The adsorption of creatinine on MIL-100(Fe) reached equilibrium in less than 2 h even at a high concentration of 200 mg L−1 for creatinine, indicating the fast adsorption of creatinine on MIL-100(Fe) (Fig. 1a). The quantitative kinetic order and adsorption rate constant for the adsorption of creatinine on MIL-100(Fe) were further determined (Fig. 1b). The results show that the adsorption of creatinine on MIL-100(Fe) was well described by a pseudo-second-order kinetics model (Table S1, ESI†).
The adsorption isotherms of creatinine on MIL-100(Fe) are shown in Fig. 2. The adsorption capacity of creatinine increased steadily with initial creatinine concentration, showing the favourable adsorption of creatinine on MIL-100(Fe) at higher concentrations, which is favourable for MIL-100(Fe) to adsorb and remove creatinine with high concentration from the blood of patients with kidney disease. To evaluate the maximum adsorption capacity of MIL-100(Fe) for creatinine, the adsorption isotherms were fitted with Langmuir equation (Fig. 2, Table 1). Higher temperature gave larger adsorption capacity of the creatinine, indicating the adsorption of creatinine on MIL-100(Fe) was endothermic. The adsorption capacities at physiological temperature (37 °C) for creatinine on MIL-100(Fe) was 190.5 mg g−1, which is much higher than those of previous reported sorbents (Table S2, ESI†), revealing the great potential of MIL-100(Fe) for creatinine adsorption.
 | ||
| Fig. 2 Adsorption of creatinine (in PBS solution, 1 mM, pH 7.4) on MIL-100(Fe) (15 mg): (a) adsorption isotherms. (b) Corresponding Langmuir plots. | ||
| Langmuir parameters | Thermodynamic parameters | ||||||
|---|---|---|---|---|---|---|---|
| T (°C) | R2 | Q0 (mg g−1) | b (L mol−1) | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | R2 |
| 30 | 0.989 | 175.1 ± 3.6 | 116.2 ± 8.9 | −12.0 ± 0.1 | 4.7 ± 0.1 | 55.1 ± 0.5 | 0.996 |
| 37 | 0.994 | 190.5 ± 6.2 | 120.2 ± 2.1 | −12.4 ± 0.1 | |||
| 45 | 0.991 | 210.9 ± 9.7 | 125.5 ± 1.7 | −12.8 ± 0.2 | |||
| 53 | 0.992 | 215.5 ± 8.0 | 131.8 ± 5.2 | −13.3 ± 0.1 | |||
| 60 | 0.983 | 240.9 ± 13.2 | 136.9 ± 3.5 | −13.7 ± 0.1 | |||
The thermodynamic parameters, free energy change (ΔG, kJ mol−1), enthalpy change (ΔH, kJ mol−1) and entropy change (ΔS, J mol−1 K−1) for the adsorption of creatinine were determined from their Langmuir and van't Hoff plots (Table 1; Fig. S2, ESI†). The negative values of ΔG suggest spontaneous adsorption of creatinine on MIL-100(Fe) (Table 1). The positive value of ΔH confirms the endothermic process for the adsorption of creatinine on MIL-100(Fe), agreeing with the increase of adsorption capacity with increasing temperature (Table 1 and Fig. 2).
Comparison of the adsorption capacities of MOFs with different pore and window size was then used to examine if the size effect was involved in the creatinine adsorption on MIL-100(Fe) (Table S3, ESI†). MOFs with too larger or smaller pore window than creatinine (7.1 × 8.1 × 3.0 Å)6 show low adsorption capacities for creatinine, while MIL-100(Fe) with the comparable pore window (8.6 Å cf. 8.1 Å) to creatinine shows the best performance for creatinine. The results reveal the size effect of MIL-100(Fe) was involved in the high efficient adsorption of creatinine. Besides, the interactions between the Lewis base nitrogen sites of creatinine and the Lewis acid Fe sites of MIL-100(Fe) should also be considered.
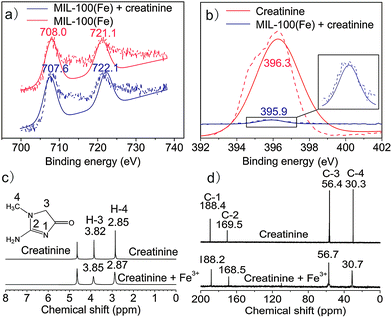
To show the coordination interactions between the Lewis base nitrogen sites of creatinine and the Lewis acid Fe sites of MIL-100(Fe), the X-ray photoelectron spectroscopy (XPS) studies were performed (Fig. 3a and b). The Fe 2p peaks of Fe at 708.0 eV and 721.1 eV for MIL-100(Fe) were shifted to 707.6 eV and 722.1 eV respectively, after the adsorption of creatinine. Likewise, the N 1s peak at 396.3 eV for creatinine was shifted to 395.9 eV after adsorption on MIL-100(Fe). These results suggest the weak coordination interactions between the Lewis base nitrogen sites on creatinine and the Lewis acid Fe sites on MIL-100(Fe).
The 1H-NMR and 13C-NMR spectra were further used to reveal the interaction sites on creatinine (Fig. 3c and d). 1H-NMR experiments show no significant change in the chemical shift of the H-3 and H-4 for creatinine after the addition of Fe3+, indicating the nitrogen between H-3 and H-4 of the creatinine should not be the interaction sites to Fe3+. For 13C-NMR, the chemical shift of C-2 decreased from 169.5 ppm to 168.5 ppm after the addition of Fe3+. The combined results of XPS and NMR show that the nitrogen on C-2 should be the interaction sites of creatinine to Fe3+.
To investigate the potential of MIL-100(Fe) for creatinine adsorption under physiological conditions, the effects of pH on the adsorption capacity of creatinine was studied (Fig. 4a). No significant effect on the adsorption capacity of MIL-100(Fe) for creatinine was observed in the pH range of 5 to 8, showing the potential of MIL-100(Fe) for creatinine adsorption under physiological pH (about 7.4).
To further study the potential of MIL-100(Fe) for creatinine adsorption in real samples, the adsorption of creatinine on MIL-100(Fe) was performed in Tyrode's solution (pH 7.4),4 a solution mimicking the mineral composition and pH of blood. It is very encouraging that the adsorption kinetic and capacity of MIL-100(Fe) for creatinine in Tyrode's solution is comparable to that in PBS (pH 7.4) solution (Fig. 1a). However, the adsorption capacity of creatinine decreased in the presence of human serum albumin (HSA) (Table 2). The decrease in the adsorption capacity resulted from the competitive adsorption of HSA on the surface of MIL-100(Fe). Although the equilibrium adsorption capacity of creatinine on MIL-100(Fe) decreases to 13.6 mg g−1 in the presence of HSA (40 mg mL−1), it is still higher than the maximum adsorption capacity of some reported sorbents in the absence of HSA (Table S2, ESI†).
| Concentration of HSA (mg mL−1) | qe (mg g−1) |
|---|---|
| 0 | 29.57 ± 0.34 |
| 20 | 18.62 ± 1.06 |
| 40 | 13.65 ± 0.81 |
Facile regeneration of an adsorbent is very important not only for its environmental friendliness but also for its commercial feasibility. 80% of adsorbed creatinine was desorbed from the MIL-100(Fe) with 1 mL methanol under 5 min ultrasonication (Fig. 4b). Repeating of such regeneration step for three times allowed 97.6% of creatinine to be desorbed (Fig. S3, ESI†). The regenerated MIL-100(Fe) could be used for the adsorption of creatinine repeatedly without a significant loss of the adsorption capacity (Fig. 4c). In addition, the MIL-100(Fe) frameworks did not collapse after repeated adsorptions of creatinine and regenerations with methanol (Fig. 4d). The above results show the excellent reusability of MIL-100(Fe) for creatinine adsorption.
In summary, we have demonstrated the novel application of MOFs as artificial kidney to remove a typical uremic toxin creatinine in view of the kinetics, thermodynamics, adsorption isotherms, and reusability. MIL-100(Fe) not only shows high adsorption capacity, but also offers excellent reusability for creatinine. These features make MIL-100(Fe) potential for the adsorption and removal of creatinine. Further investigations should pay more attention to the fabrication of MOFs films or MOFs-based dialysis membranes for hemodialysis.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants 21305071 and 21275079), Tianjin Natural Science Foundation (Grants 14JCZDJC37600 and 14JCQNJC06600), and the Fundamental Research Funds for the Central Universities.Notes and references
- R. Vanholder, R. De Smet, G. Glorieux, A. Argiles, U. Baurmeister, Ph. Brunet, W. Clark, G. Cohen, P. P. De Deyn, R. Deppisch, B. Descamps-Latscha, T. Henle, A. Jorres, H. D. Lemke, Z. A. Massy, J. Passlick-Deetjen, M. Rodriguez, B. Stegmayr, P. Stenvinkel, C. Tetta, Ch. Wanner and W. Zidek, Kidney Int., 2003, 63, 1934 CrossRef CAS PubMed.
- M. Wyss and R. Kaddurah-Daouk, Physiol. Rev., 2000, 80, 1107 CAS.
- B. Gao, Y. Yang, J. Wang and Y. Zhang, J. Biochem. Mol. Toxicol., 2008, 166 CrossRef CAS PubMed.
- X. P. Deng, T. Wang, F. Zhao, L. J. Li and C. S. Zhao, J. Appl. Polym. Sci., 2007, 103, 1085 CrossRef CAS PubMed.
- D. Berge-Lefranc, H. Pizzala, R. Denoyel, V. Hornebecq, J. Berge-Lefranc, R. Guieu, P. Brunet, H. Ghobarkar and O. Schäf, Microporous Mesoporous Mater., 2009, 119, 186 CrossRef CAS PubMed.
- V. Wernert, O. Schäf, H. Ghobarkar and R. Denoyel, Microporous Mesoporous Mater., 2005, 83, 101 CrossRef CAS PubMed.
- D. Berge-Lefranc, O. Schäf, R. Denoyel, J. Berge-Lefranc, R. Guieu, P. Brunet and V. Hornebecq, Microporous Mesoporous Mater., 2010, 129, 144 CrossRef CAS PubMed.
- C. Ye, Q. M. Gong, F. P. Lu and J. Liang, Sep. Purif. Technol., 2007, 58, 2 CrossRef CAS PubMed.
- Y. S. Chang, T. H. Ko, T. J. Hsu and M. J. Syu, Anal. Chem., 2009, 81, 2098 CrossRef CAS PubMed.
- H. A. Tsai and M. J. Syu, Anal. Chim. Acta, 2005, 539, 107 CrossRef CAS PubMed.
- H. A. Tsai and M. J. Syu, Biomaterials, 2005, 26, 2759 CrossRef CAS PubMed.
- M. Eddaoudi, D. B. Moler, H. L. Li, B. L. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319 CrossRef CAS PubMed.
- G. Férey, C. Mellot-Draznieks, C. Serre and F. Millange, Acc. Chem. Res., 2005, 38, 217 CrossRef PubMed.
- Z.-Y. Gu, C.-X. Yang, N. Chang and X.-P. Yan, Acc. Chem. Res., 2012, 45, 734 CrossRef CAS PubMed.
- N. A. Khan, Z. Hasan and S. H. Jhung, J. Hazard. Mater., 2013, 244–245, 444 CrossRef CAS PubMed.
- S.-H. Huo and X.-P. Yan, J. Mater. Chem., 2012, 22, 7449 RSC.
- C.-X. Yang and X.-P. Yan, J. Mater. Chem., 2012, 22, 17833 RSC.
- C.-Y. Huang, M. Song, Z.-Y. Gu, H.-F. Wang and X.-P. Yan, Environ. Sci. Technol., 2011, 45, 4490 CrossRef CAS PubMed.
- C.-X. Yang and X.-P. Yan, Chin. J. Anal. Chem., 2013, 41, 1297 CAS.
- P. Horcajada, S. Surble, C. Serre, D. Y. Hong, Y. K. Seo, J. S. Chang, J. M. Greneche, I. Margiolaki and G. Férey, Chem. Commun., 2007, 2820 RSC.
- A. C. McKinlay, R. E. Morris, P. Horcajada, G. Férey, R. Gref, P. Couvreur and C. Serre, Angew. Chem., Int. Ed., 2010, 49, 6260 CrossRef CAS PubMed.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J. S. Chang, Y. K. Hwang, V. Marsaud, P. N. Bories, L. Cynober, S. Gil, G. Férey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172 CrossRef CAS PubMed.
- Z.-Y. Gu, Y.-J. Chen, J.-Q. Jiang and X.-P. Yan, Chem. Commun., 2011, 47, 4787 RSC.
- J.-Q. Jiang, C.-X. Yang and X.-P. Yan, ACS Appl. Mater. Interfaces, 2013, 5, 9837 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05111d |
| This journal is © The Royal Society of Chemistry 2014 |