Simultaneous dehydration of biomass-derived sugars to 5-hydroxymethyl furfural (HMF) and reduction of graphene oxide in ethyl lactate: one pot dual chemistry†
Dibyendu Mondalab,
Jai Prakash Chaudharyab,
Mukesh Sharmaab and
Kamalesh Prasad*ab
aMarine Biotechnology and Ecology Discipline, CSIR-Central Salt and Marine Chemicals Research Institute (CSIR-CSMCRI), Council of Scientific & Industrial Research (CSIR), Gijubhai Badheka Marg, Bhavnagar-364 002, Gujarat, India. E-mail: kamlesh@csmcri.org; drkamaleshp@gmail.com; Fax: +91-278-2567562; Tel: +91-278-2567760
bAcademy of Scientific and Innovative Research(AcSIR)-Central Salt and Marine Chemicals Research Institute, Gijubhai Badheka Marg, Bhavnagar-364 002, Gujarat, India
First published on 12th June 2014
Abstract
Low yield of chemicals is often identified as a major obstacle for the complete utilization of bioresources as a source of important chemicals and thereby limits their application in industries. The issue of low yield can be partially compensated by integrated processes, i.e., production of two or more chemicals from the same biomass using single or multistep processes. Herein, a simple pathway for simultaneous production of 5-hydroxymethyl furfural (HMF) from biomass-derived sugars by dehydration of fructose (molar yield 76.3%) using graphene oxide (GO) as acid catalyst and choline chloride (ChoCl) as additive in ethyl lactate is demonstrated. Moreover, during the course of reaction GO was reduced to produce six-layered graphene nanosheets (96% recovery). Furthermore, the solvent was recycled after recovery of both products and successfully reused for subsequent production of the two chemicals with high purity.
Introduction
Biomass is considered the most appropriate sustainable resource for the production of biofuels as alternative to existing fuels derived from fossil resources. Research has geared up recently due to the gradual dwindling of fossil resources. It is proposed that 18% of total manufactured chemicals will be sourced from biomass by 2020.1 Although there are plenty of reports available in the literature on conversion of biomass to ethanol or other valuable chemicals,2 special attention has been focused on 5-hydroxymethylfurfural (HMF), which is the dehydrated product of simple sugars and is the precursor of the superior biofuel, 2,5-dimethylfuran (DMF).3–5 Usually, HMF is prepared from fructose, glucose, sucrose, cellulose and inulin.3 In this line of research, we have recently demonstrated Kappaphycus alvarezii, a red seaweed, as an effective alternative precursor for the production of HMF along with potassic fertilizer, levulinic acid, formic acid and pure water.5 HMF is considered an important platform chemical, required in different biorefinery processes to derive various important chemicals as shown in Scheme S1 (ESI†).2 Although several routes for the synthesis of HMF are reported in the literature, including processes using ionic liquids and other solid acid catalysts,6,7 there are only few reports available on using bio-based catalyst for such syntheses. Recently, it was reported that HMF could be produced with a maximum yield of 84% using choline chloride (ChoCl)/betaine hydrochloride (BHC)/water system at 110 °C under heating for 1 h.8 Use of ChoCl–H2O/MIBK (methyl isobutyl ketone) biphasic system in the presence of AlCl3 catalyst at 165 °C under microwave condition for 15 minute produced HMF with 70% yield.9,10Carbon-based materials such as carbon nanotubes and graphene oxide (GO) have been explored as green catalysts for a number of sustainable chemical transformations.11,12 GO was reported as an efficient catalyst for dehydration of fructose to HMF (31% yield) at 100 °C for 24 h.13 The produced HMF was further reacted with ethanol to yield ethoxymethyl furfural but the fate of GO was not discussed, i.e., whether GO was reduced during the reaction was not investigated.13
While HMF is no doubt of great importance, it is of immense interest that the economics of renewable bioresources can be made more attractive through integration with the conformation of other essential products having practical commercial applications.14 Carbon-based nano materials, especially graphene, is going to play a very vital role in the development of new materials in the future. Graphene consists of 2D sheet-like structure with “honeycomb” decoration and made up of conjugated sp2 carbon.15 Graphene is used extensively for energy storage,16 and as membranes for separation of gases and for desalination.17,18 Chemical reduction of GO is one potential route to prepare large scale graphene. To date, there have been 50 types of different reducing agents used for the reduction of GO to graphene.15 The most commonly used methodology is reduction using hydrazine or hydrazine derivatives.19,20 The disadvantage of using such reducing agents is their toxicity and hence immense care is required during reaction to avoid contamination. Poly (diallyldimethylammonium chloride) (PDDA) containing quaternary ammonium salts, ammonia, glucose, fructose and sucrose are reported as effective alternative reducing agents for GO.21–23
Thus, keeping all the above literature studies in mind, we proposed for the first time the use of GO, simple sugars, and choline chloride or betaine hydrochloride in one pot using ethyl lactate (EL) as solvent for the simultaneous synthesis of HMF and reduced graphene oxide nanosheets (rGO) (Scheme 1). Ethyl lactate was chosen as a solvent for the following reasons: (i) it can be derived from biomass as shown in Scheme S2 (ESI†),24 which makes entire process bio-based; (ii) it is an environment friendly and green alternative to petrochemical-derived solvents; and (iii) it has been reported as a bio-based solvent for synthesis of aryl aldimines,25 disulfide26 and other reactions. However, the potential of this solvent has been unexplored to date for the synthesis of HMF and rGO. Moreover, the dehydration efficiency of sugars to HMF is dependent on the nature of the solvent employed. Particularly, anhydrous solvent systems, which can dissolve sugar in high quantity, are useful since they prevent the side reaction of HMF degradation to levulinic acid and formic acid. Accordingly, it was worthwhile to select EL as solvent.
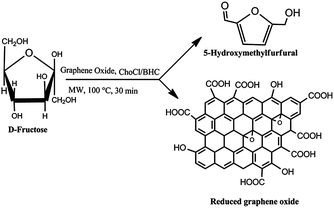 | ||
| Scheme 1 Simultaneous production of HMF and reduced graphene oxide from fructose promoted by graphene oxide using choline chloride and betaine hydrochloride as additives. | ||
Experimental section
Synthesis of graphene oxide (GO)
Graphene oxide was synthesized by a previously reported procedure.27 In a typical experiment, 0.5 g of graphite nano powder was taken in a RBF containing 25 mL of 98% H2SO4. The RBF was fitted in an ice bath to maintain a temperature of 0 to 4 °C. 0.5 g of sodium nitrate was added into the RBF, followed by slow addition of 3 g of potassium permanganate (KMnO4), with constant stirring at 0–4 °C. After that, the above mixture was transferred to a water bath maintained at 35 ± 5 °C and stirred for another 1 h. Then, 40 mL of Milli-Q water was slowly added, and the temperature was raised to 90 ± 5 °C for 30 min with continuous stirring. Finally, 100 mL of Milli-Q water was added, followed by slow addition of 3.0 mL of H2O2 (30% v/v). When the colour of the solution changed from dark brown to yellowish, it was centrifuged and the residue was washed first with 5% HCl, and then 0.3% H2O2 was added, followed by addition of pure Milli-Q water to remove all impurities from the oxidized graphite. Finally, the residue was collected and freeze-dried to obtain GO.Simultaneous synthesis of HMF and reduced graphene oxide (rGO)
In an optimized reaction procedure, 250 mg of fructose was taken in a 50 mL round-bottomed flask followed by the addition of 10 mL of EL, 100 mg of ChoCl and 10 mg of GO. The RBF was placed under a microwave synthesizer (Star-S, Milestone, Italy) and the reaction mixture was irradiated with 600 W at 100 °C for 30 min. After that, the reaction mixture was allowed to cool to room temperature and was processed further as described below.The above reaction mixture was centrifuged to separate GO from the solution. The recovered GO was washed several times with Milli-Q water followed by centrifugation, and three times with acetone followed by centrifugation. After that, the washed GO was dried in the oven overnight. The total recovery of modified GO was 96% (w/w) with respect to the GO taken initially, and the reduction of GO was confirmed primarily by UV measurement.
A small portion of the solution (20 μL) after recovery of rGO was diluted with HPLC grade water (1.0 mL) for HPLC measurements to confirm formation of HMF and to quantify it. The other portion of the solution, containing EL, ChoCl and HMF, was evaporated under vacuum to separate as much as EL (90% recovery) from the solution, and the resultant concentrated solution was diluted with 10 mL Milli-Q water and HMF was extracted from this solution by methyl isobutyl ketone (MIBK). After removing MIBK, HMF was obtained with 98% purity as confirmed by HPLC.
For the simultaneous synthesis of HMF and rGO in one pot using BHC as additive, the same procedure was followed as mentioned above, except 100 mg BHC was taken instead of 100 mg ChoCl. For the synthesis of HMF and rGO from other sugar substrates (glucose, galactose, mannose, sucrose), the same procedure was followed as described above, except 250 mg of respective sugar was taken as a substitute for fructose.
Results and discussion
Various sugars, namely, D-fructose, D-glucose, D-mannose, D-galactose and D-sucrose, were used as substrates for the production of HMF using ethyl lactate as solvent under microwave irradiation (MW). GO played a role as acid catalyst13 and ChoCl and BHC were used as additives in the process (Fig. S1, ESI†). As shown in Table 1, various reaction parameters were optimized to achieve maximum quantity of HMF from fructose. It can be seen that dehydration of only fructose in EL did not give formation of HMF, whereas highest molar yield of HMF (76.3%) was achieved from the dehydration of 2.5% w/v fructose, 0.1% w/v of GO, 1% w/v of ChoCl at 100 °C for 30 min under MW irradiation. Moreover, HMF up to 69.6% molar yield was achieved using BHC (entry 13, Table 1). However, only 21.7% molar yield of HMF was achieved in the absence of GO, indicating that it worked as a catalyst in the process. Furthermore, at 0.5% concentration of additive (ChoCl & BHC) the yield of HMF was 53% and 64% respectively. Upon increasing additive concentration up to 1% the yield of HMF increased significantly and further increment of additive concentration resulted in decrease in HMF yield as can be seen in the ESI†, Fig. S2. At higher concentration of additive, HMF may degrade to levulinic acid, which is responsible for the observed reduction in the HMF yield. Taking inference from these observations, the reaction parameters of entry 9 in Table 1 was chosen as optimized reaction conditions for the production of HMF from fructose (Scheme 1).| Entrya | Fructose concentration (% w/v) | GO (% w/v) | ChoCl (% w/v) | BHC (% w/v) | Ethyl lactate (mL) | Reaction temperature (°C) | Reaction time (min) | Molar yield of HMFb (%) |
|---|---|---|---|---|---|---|---|---|
| a Reaction condition = microwave irradiation, 600 W power.b Yields are based on HPLC quantification. GO = graphene oxide; ChoCl = choline chloride; BHC = betaine hydrochloride. | ||||||||
| 1 | 2.5 | — | — | — | 10.0 | 100 | 30 | 0.0 |
| 2 | 2.5 | 0.1 | — | — | 10.0 | 100 | 30 | 12.0 |
| 3 | 2.5 | — | 1.0 | — | 10.0 | 100 | 30 | 18.4 |
| 4 | 2.5 | — | — | 1.0 | 10.0 | 100 | 30 | 21.7 |
| 5 | 2.5 | 0.1 | 1.0 | — | 10.0 | 100 | 10 | 61.5 |
| 6 | 2.5 | 0.1 | 1.0 | — | 10.0 | 100 | 20 | 65.8 |
| 7 | 2.5 | 0.1 | 1.0 | — | 10.0 | 80 | 30 | 51.5 |
| 8 | 2.5 | 0.1 | 1.0 | — | 10.0 | 90 | 30 | 58.9 |
| 9 | 2.5 | 0.1 | 1.0 | — | 10.0 | 100 | 30 | 76.3 |
| 10 | 2.5 | 0.1 | 1.0 | — | 10.0 | 110 | 30 | 60.0 |
| 11 | 2.5 | 0.1 | — | 1.0 | 10.0 | 80 | 30 | 55.6 |
| 12 | 2.5 | 0.1 | — | 1.0 | 10.0 | 90 | 30 | 64.9 |
| 13 | 2.5 | 0.1 | — | 1.0 | 10.0 | 100 | 30 | 69.6 |
| 14 | 2.5 | 0.1 | — | 1.0 | 10.0 | 110 | 30 | 54.8 |
It can be observed from Fig. 1 that the yield of HMF was highest when fructose was used as substrate, followed by glucose, sucrose, mannose and galactose. The least conversion in the case of galactose is due to the dehydration of the sugar to tagatose,4 unlike the rest of the sugars, which dehydrated to HMF (Scheme S3, ESI†). Furthermore, it was observed that the additives (ChoCl and BHC) affected the yield of the produced HMF (Fig. S2, ESI†). When 1% of ChoCl was used, the highest HMF produced was 76.3%, whereas production was 69% with a similar amount of BHC. The HMF formed was quantified using HPLC, and chromatograms obtained with various reaction parameters are shown in Fig. S3 (ESI†). EL used in the process was recycled, and in the 1H NMR studies, the peaks of the impurities were absent, indicating the purity of the recycled solvent (Fig. S4, ESI†), which was successfully reused for HMF production.
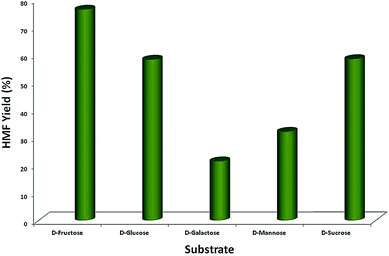 | ||
| Fig. 1 Effect of substrate concentration on HMF yield under MW irradiation (600 W) at 100 °C using GO as acid catalyst, EL as solvent. | ||
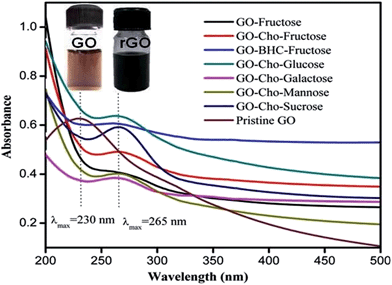
GO present in the above reaction mixture after completion of reaction was isolated by ultracentrifugation and purified by repeated washing with water and acetone (total recovery of modified GO was 96% w/w with respect to GO taken initially). The brownish colour solution containing GO turned black upon above reaction as shown in Fig. 2, showing visual evidence for the conversion of GO to rGO. Due to the π–π transitions of aromatic C–C bonds present in GO, an absorbance at λmax = 230 nm was observed in pristine GO. Different GO samples which were recovered during the course of HMF synthesis showed λmax = ∼265 nm. This red shifted absorbance maxima indicated the reduction of pristine GO (Fig. 2).27 Although for all samples the λmax = 230 nm was shifted to λmax = 265 nm, improved reduction was observed for systems in which ChoCl was used as the additive and sucrose, fructose and glucose were used as substrates (rGO–ChoCl–sucrose, rGO–ChoCl–fructose and rGO–ChoCl–glucose). (Please refer to Table S1 (ESI†) for the abbreviations.)
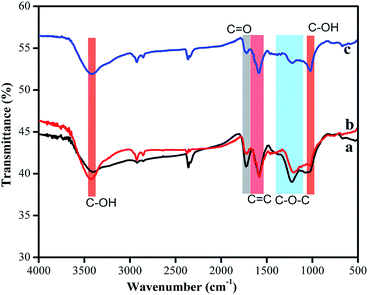
The reduction of GO during the course of HMF synthesis was also confirmed by FT-IR spectra (Fig. 3). Various oxygen functionalities in the GO structure were observed in FT-IR spectra. The bands at 3437 cm−1 and 1040 cm−1 can be ascribed to C–OH vibration, whereas the bands at 1729 cm−1 are due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of carbonyl of COOH group. The peak positions at 1627 cm−1 and 1580 cm−1 are due to sp2 hybridized in-plane stretching vibration and the absorption bands at 1360 cm−1 and 1220 cm−1, which are responsible for C–O–C stretching vibration of epoxide functionalities present in the GO moiety.28 As can be seen from the figure, the peak positions for C
O stretching vibration of carbonyl of COOH group. The peak positions at 1627 cm−1 and 1580 cm−1 are due to sp2 hybridized in-plane stretching vibration and the absorption bands at 1360 cm−1 and 1220 cm−1, which are responsible for C–O–C stretching vibration of epoxide functionalities present in the GO moiety.28 As can be seen from the figure, the peak positions for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O–C reduced significantly in the case of GO obtained from the GO–ChoCl–fructose sample as compared to GO obtained from GO–fructose sample. FT-IR spectra for reduced GO in presence of sucrose and pristine GO are shown in Fig. S5 and S6 (ESI†), respectively.
O and C–O–C reduced significantly in the case of GO obtained from the GO–ChoCl–fructose sample as compared to GO obtained from GO–fructose sample. FT-IR spectra for reduced GO in presence of sucrose and pristine GO are shown in Fig. S5 and S6 (ESI†), respectively.
X-ray diffraction (XRD) patterns of pristine GO exhibited a characteristic peak of (001) at 2θ = 11.29°, having interlayer spacing of 7.68 Å, which ensures the presence of oxygen functionality after oxidation of graphite. The peaks of (002) at 2θ = 26.46° and (010) at 2θ = 42.18° are due to the original graphite peaks.29 The XRD pattern of GO (GO–fructose) obtained after HMF synthesis using GO as catalyst showed a new peak broadening to (002) at 2θ = 22.96°, having much lower interlayer spacing of ∼4.40 Å, but the peak of (001) at 2θ = 11.29° with interlayer spacing ∼7.78 Å also remained, which indicated only partial reduction of GO (Table 2). A similar trend was observed for GO obtained during HMF synthesis from fructose using BHC as additive (GO–BHC–fructose) (Fig. S7, ESI†). The GO obtained during HMF synthesis using ChoCl as additive showed mainly peak broadening of (002) at 2θ = 25.41°, having a d-spacing = 3.9 Å (0.39 nm), which indicates complete reduction of GO (Fig. S8, ESI†).
| Sample ID | d-Spacing (Å) | Reference |
|---|---|---|
| Pristine GO | 7.68 | Present study |
| Hydrazine–reduced GO | 3.86 | Park et al., 2009 (ref. 31) |
| GO–fructose | 4.40 | Present study |
| GO–BHC–fructose | 4.00 | Present study |
| GO–ChoCl–fructose | 3.91 | Present study |
| GO–ChoCl–glucose | 4.17 | Present study |
| GO–ChoCl–galactose | 3.94 | Present study |
| GO–ChoCl–mannose | 4.02 | Present study |
| GO–ChoCl–sucrose | 4.14 | Present study |
To further confirm the reduction of GO, micro-Raman spectra of the samples were recorded. The peak for HGO at G band (1594 cm−1) was down shifted to 1583 cm−1, indicating reduction of GO (Fig. 4).30 Furthermore, HGO exhibited D/G intensity ratio of 0.87, and after the reaction the ratio was changed to 0.94, indicating formation of more crystalline domains after reduction and supported the reduction of GO described above.
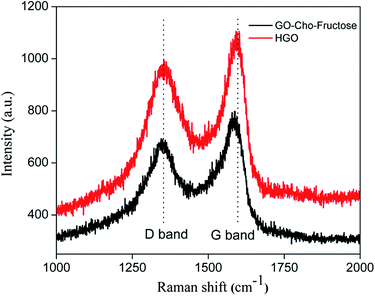 | ||
| Fig. 4 Raman spectra of Hummers GO (red) and GO reduced in presence of fructose and choline chloride (black). | ||
The loss of CO, CO2 and water molecule from carboxylic and hydroxyl functionalities present in the GO sheets occurred in the temperature range from ∼120 °C to ∼265 °C (Fig. S9, ESI†). In this temperature range, 30.8% and 18.5% weight loss were observed for GO–fructose and GO–BHC–fructose, respectively, which indicated partial reduction of GO as observed in XRD analysis. The weight loss of 11.0% and 9.6% were observed in the abovementioned temperature range for GO–ChoCl–fructose and GO–ChoCl–sucrose, respectively, which confirmed almost complete reduction of GO for these samples.
TEM images of GO prepared by Hummers method and reduced by hydrazine showed presence of 32 layers with interlayer distance of 0.39 nm (Fig. S10, ESI†). The GO reduced using fructose as substrate and without any additive showed presence of 19 layers with interlayer distance of 0.36 nm (Fig. 5a and b). However, GO reduced using ChoCl as additive and fructose as substrate showed presence of 6 layers with interlayer distance of 0.34 nm (Fig. 5d and e). The lower number of sheets is due to exfoliation of rGO in presence of additive. The presence of ChoCl facilitated formation of rGO with few layers. Due to the importance of few-layered graphene in applications such as selective gas separation,32 it is considered of immense interest to prepare graphene having single or few layers. However, the rGO obtained for other sugars such as galactose, sucrose and mannose had 12, 14 and 17 layers, respectively (Fig. S11a–c, ESI†). Moreover, when BHC was used as additive and fructose was used as substrate, 10-layered rGO was formed (Fig. S12, ESI†).
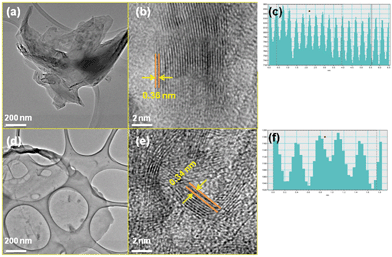 | ||
| Fig. 5 TEM images (a, b and c) graphene oxide reduced in presence of fructose and (d, e and f) graphene oxide reduced in presence of fructose and choline chloride. | ||
A tertiary ammonium salt, diallyldimethylammonium chloride (PDDA), has been reported to adsorb through π–π and electrostatic interactions, which induces electrostatic repulsion between graphene nanosheets and prohibits aggregation in aqueous suspensions.21 It was hypothesized that the inherent positive charge of PDDA, containing N+ groups, may hit the oxygen atom of epoxide in graphene oxide, which stimulates a ring-opening reaction, facilitating a lone electron on the α-carbon and subsequent elimination of the nitroso group to afford formation of an olefin (Scheme 2). In a similar manner, ChoCl and BHC used in the present study interacted with the nanosheets of GO, resulting in electrostatic repulsion among them and perhaps is the reason for the lowering of the number of sheets of rGO (Scheme S2†). However, BHC exists in zwitterionic form in solution (Fig. S13, ESI†). The presence of anion in the structure resulted in a dual competitive interaction with GO; hence, the reduction efficiency of graphene oxide was less when BHC was used as additive instead of ChoCl and resulted in the formation of more layers (10 layers).
Furthermore, it is reported that simple sugars like glucose have the ability to form a chemical bond with the carbon of GO as shown in Fig. S6 (ESI†).23 This type of chemical bonding is similar to phosphate group binding with glucose.33 Moreover, this chemical bonding between sugar and GO carbon resulted in a steric interaction, which prevented a π–π stacking interaction among the graphene sheets and promoted the conversion of a stacking GO structure to few-layered GO nanosheets.
Conclusions
In summary, we have demonstrated a facile integrated pathway for simultaneous dehydration of simple sugars to 5-hydroxymethyl furfural and reduction of graphene oxide, which was used as acid catalyst in the process in a bio-based green solvent, ethyl lactate. The solvent was recycled and successfully reused in the process. The molar yield of HMF was as high as 76.3% when fructose was used as substrate and choline chloride as additive. The reduced graphene oxide obtained herein had a lower number of sheets in comparison to GO reduced by conventional method.Acknowledgements
CSIR-CSMCRI communication no. 028/14. KP thanks CSIR, New Delhi, for the grant of CSIR-Young Scientist Awardees and CSIR-EMPOWER Project and for overall financial support. DM and MS thank CSIR and UGC for Junior Research Fellowships. JPC thanks DST, New Delhi, for fellowship. Dr S. K. Nataraj is acknowledged for useful discussions. DM, MS and JPC are also thankful to AcSIR for enrollment in Ph.D. and to “Analytical Discipline and Centralized Instrumental Facilities” for providing instrumentation facilities.Notes and references
- Biomass Research and Development Technical Advisory Committee, Roadmap for Biomass Technologies in the U.S., U.S. Government, 2002, http://www.usbiomassboard.gov/pdfs/final_biomass_roadmap_200kw.pdf.
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC.
- Y. Román-Leshkov, C. J. Barrett, Z. Y. Liu and J. A. Dumesic, Nature, 2007, 447, 982–986 CrossRef PubMed.
- J. B. Binder, A. V. Cefali, J. J. Blank and R. T. Raines, Energy Environ. Sci., 2010, 3, 765–771 CAS.
- D. Mondal, M. Sharma, P. Maiti, K. Prasad, R. Meena, A. K. Siddhanta, P. Bhatt, S. Ijardar, V. P. Mohandas, A. Ghosh, K. Eswaran, B. G. Shah and P. K. Ghosh, RSC Adv., 2013, 3, 17989–17997 RSC.
- O. O. James, S. Maity, L. A. Usman, K. O. Ajanaku, O. O. Ajani, T. O. Siyanbola, S. Sahu and R. Chaubey, Energy Environ. Sci., 2010, 3, 1833–1850 CAS.
- D. J. Liu and E. Y.-X. Chen, Green Chem., 2014, 16, 964–981 RSC.
- K. D. O. Vigier, A. Benguerba, J. Barrault and F. Jérôme, Green Chem., 2012, 14, 285–289 RSC.
- F. Liu, J. Barrault, K. D. O. Vigier and F. Jérôme, ChemSusChem, 2012, 5, 1223–1226 CrossRef CAS PubMed.
- F. Liu, M. Audemar, K. D. O. Vigier, D. Cartigny, J.-M. Clacens, M. F. C. Gomes, A. A. H. Pádua, F. D. Campo and F. Jérôme, Green Chem., 2013, 15, 3205–3213 RSC.
- D. S. Su, J. Zhang, B. Frank, A. Thomas, X. C. Wang, J. Paraknowitsch and R. Schlögl, ChemSusChem, 2010, 3, 169–180 CrossRef CAS PubMed.
- C. Huang, C. Li and G. Shi, Energy Environ. Sci., 2012, 5, 8848–8868 CAS.
- H. Wang, T. Deng, Y. Wang, X. Cui, Y. Qi, X. Mu, X. Hou and Y. Zhu, Green Chem., 2013, 15, 2379–2383 RSC.
- S. G. Wettstein, D. M. Alonso, Y. Chong and J. A. Dumesic, Energy Environ. Sci., 2012, 5, 8199–8203 CAS.
- C. K. Chua and M. Pumera, Chem. Soc. Rev., 2014, 43, 291–312 RSC.
- C. Xu, B. Xu, Y. Gu, Z. Xiong, J. Sun and X. S. Zhao, Energy Environ. Sci., 2013, 6, 1388–1414 CAS.
- H. Du, J. Li, J. Zhang, G. Su, X. Li and Y. Zhao, J. Phys. Chem. C, 2011, 115, 23261–23266 CAS.
- D. Cohen-Tanugi and J. C. Grossman, Nano Lett., 2012, 12, 3602–3608 CrossRef CAS PubMed.
- V. C. Tung, M. J. Allen, Y. Yang and R. B. Kaner, Nat. Nanotechnol., 2009, 4, 25–29 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS PubMed.
- S. Zhang, Y. Shao, H. Liao, M. H. Engelhard, G. Yin and Y. Lin, ACS Nano, 2011, 5, 1785–1791 CrossRef CAS PubMed.
- C. Zhu, S. Guo, Y. Fang and S. Dong, ACS Nano, 2010, 4, 2429–2437 CrossRef CAS PubMed.
- O. Akhavan, E. Ghaderi, S. Aghayee, Y. Fereydoonia and A. Talebi, J. Mater. Chem., 2012, 22, 13773–13781 RSC.
- C. S. M. Pereira, V. M. T. M. Silva and A. E. Rodrigues, Green Chem., 2011, 13, 2658–2671 RSC.
- J. S. Bennett, K. L. Charles, M. R. Miner, C. F. Heuberger, E. J. Spina, M. F. Bartels and T. Foreman, Green chem, 2009, 11, 166–168 RSC.
- Y. Li, H. Wang, C. Wang, J.-P. Wan and C. Wen, RSC Adv., 2013, 3, 21369–21372 RSC.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- R. Y. N. Gengler, D. S. Badali, D. Zhang, K. Dimos, K. Spyrou, D. Gournis and R. J. D. Miller, Nat. Commun., 2013, 4, 2560 Search PubMed.
- M. Acik, G. Lee, C. Mattevi, M. Chhowalla, K. Cho and Y. J. Chabal, Nat. Mater., 2010, 9, 840–845 CrossRef CAS PubMed.
- A. J. Grover, M. Z. Cai, K. R. Overdeep, D. E. Kranbuehl and H. C. Schniepp, Macromolecules, 2011, 44, 9821–9829 CrossRef.
- S. Park, J. An, I. Jung, R. D. Piner, S. J. An, X. Li, A. Velamakanni and R. S. Ruoff, Nano Lett., 2009, 9, 1593–1597 CrossRef CAS PubMed.
- H. W. Kim, H. W. Yoon, S.-M. Yoon, B. M. Yoo, B. K. Ahn, Y. H. Cho, H. J. Shin, H. Yang, U. Paik, S. Kwon, J.-Y. Choi and H. B. Park, Science, 2013, 342, 91–95 CrossRef CAS PubMed.
- R. B. Robey and N. Hay, Oncogene, 2006, 25, 4683–4696 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05049e |
| This journal is © The Royal Society of Chemistry 2014 |