Structure – chiroptical properties relationship of cisoid enones with an α-methylenecyclopentanone unit†
Jadwiga Frelek*a,
Aleksandra Butkiewicza,
Marcin Góreckia,
Ryszard K. Wojcieszczykb,
Roman Luboradzkic,
Marcin Kwitd,
Michał F. Rodee and
Wojciech J. Szczepek*f
aInstitute of Organic Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, Warsaw, Poland. E-mail: jadwiga.frelek@icho.edu.pl
bUniversity of Technology and Humanities in Radom, Chrobrego 27, 26-600 Radom, Poland
cInstitute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, Warsaw, Poland
dDepartment of Chemistry, Adam Mickiewicz University, Grunwaldzka 6, 60-780 Poznań, Poland
eInstitute of Physics, Polish Academy of Sciences, al. Lotników 32/46, 02-668 Warsaw, Poland
fPharmaceutical Research Institute, Rydygiera 8, 01-793 Warsaw, Poland
First published on 29th August 2014
Abstract
In the present work, the validity of sector and helicity rules correlating the stereostructure of cis-enones containing the 2-methylenecyclopentanone unit with the sign of the nπ* Cotton effect (CE) observed in their electronic circular dichroism (ECD) spectra is assessed. To this end, a series of model steroid cis-enones with five-membered ketone rings was synthesized. To investigate the scope and limitations of existing rules a combination of ECD spectroscopy, X-ray analysis, and time-dependent density functional theory (TD-DFT) calculations were utilized. A comparison of the experimental ECD spectra with spectra simulated by the TD-DFT calculations gave a reasonable interpretation of the nπ* CE's observed in the 360–335 nm spectral range. The results suggest that the previously articulated rules are not applicable to the investigated compounds. On the basis of comprehensive analysis of collected data, a new rule correlating perfectly the structure of studied enones with the signs of their nπ* CE was proposed. This rule correlates directly the sign of the torsion angle “b” of the cyclopentanone ring of cis-enone with the sign of the nπ* CE.
1. Introduction
The enone chromophoric system is present in a wide range of natural products that frequently display notable biological activity. To illustrate, terpenoids such as pinocarvone1 and pulegone,2 possessing this moiety, are used as flavouring agents in perfumery and aromatherapy. Another representative example, sarkomycin A,3 is cited as an antitumor agent. Members of family of guanacastepenes4 display the antibiotic activity against drug resistant strains of Staphylococcus aureus and Enterococcus faecalis while heptemerone G5,6 demonstrates the cytostatic activity.A biological activity, among other rationale, justified the high level of interest in exploration of that class of compounds, both experimentally and theoretically. The published studies of the relationship between structure and chiroptical properties of enone chromophore concerned primarily the trans-enones.7–22 In contrast, the cis-enones were examined less frequently, with focus mostly on the steroidal 6-membered compounds.7,16–20,23 These studies culminated in the formulation of handful of empirical rules correlating the sign of cotton effects (CE's) observed in the electronic circular dichroism spectra (ECD) with the absolute configuration of cis-enones.7,15,23 According to the first enone helicity rule,9–12,19,22 a positive enone torsion angle ω (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C
C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) gives rise to a negative nπ* CE and a positive ππ* CE whereas a negative enone torsion angle predicts a positive nπ* CE and a negative ππ* CE. The proposed later Kirk's enone orbital helicity rule,7,18,19,22 predicts the opposite signs of CE's compared to the enone helicity rule. The successful applicability of enone orbital helicity rule was demonstrated for many studied cis-enones with cyclohexanone ring,16,18,24 but in principle mainly for the nπ* CE's. Unexpectedly, however, the nardosinone – sesquiterpene cis-enone with cyclohexanone ring – shows a positive nπ* CE while its conformational analysis found a negative enone torsion angle.25 The analogous observation was made for the steroidal cis-enone with cyclohexanone ring.26 In such cases, the exception to the rule was explained by the dominant influence of conformation of cyclohexanone ring on the ECD spectra. It was assumed that the non-symmetrical boat conformation of this ring was the origin of abnormal chiroptical behavior within the nπ* absorption range.26 For a few examined skewed cis-enones with cyclopentanone ring15 the sign of the nπ* CE seems to be predicted properly by the enone helicity rule, whereas for cis-enones with nearly planar chromophore the octant-like contributions were expected to dominate the same transition.18,19,27
C) gives rise to a negative nπ* CE and a positive ππ* CE whereas a negative enone torsion angle predicts a positive nπ* CE and a negative ππ* CE. The proposed later Kirk's enone orbital helicity rule,7,18,19,22 predicts the opposite signs of CE's compared to the enone helicity rule. The successful applicability of enone orbital helicity rule was demonstrated for many studied cis-enones with cyclohexanone ring,16,18,24 but in principle mainly for the nπ* CE's. Unexpectedly, however, the nardosinone – sesquiterpene cis-enone with cyclohexanone ring – shows a positive nπ* CE while its conformational analysis found a negative enone torsion angle.25 The analogous observation was made for the steroidal cis-enone with cyclohexanone ring.26 In such cases, the exception to the rule was explained by the dominant influence of conformation of cyclohexanone ring on the ECD spectra. It was assumed that the non-symmetrical boat conformation of this ring was the origin of abnormal chiroptical behavior within the nπ* absorption range.26 For a few examined skewed cis-enones with cyclopentanone ring15 the sign of the nπ* CE seems to be predicted properly by the enone helicity rule, whereas for cis-enones with nearly planar chromophore the octant-like contributions were expected to dominate the same transition.18,19,27
To gain a better understanding of this exceptional behavior, we decided to thoroughly investigate set of selected β-unsubstituted, β-monosubstituted and ββ-disubstituted cis-enones, all being derivatives of cyclopentanone. The review of available literature indicated that such enones were studied in less detail than the cyclohexanone derivatives. As models for our chiroptical studies cis-enones based on the steroidal skeleton (compounds 1–3, 4a, 5, 7a,b and 8a,b) as well as commercially available jervine, i.e. 17,23β-epoxy-3β-hydroxyveratraman-11-one (compound 6), were selected (Chart 1). Our choice of these cis-enones was motivated by the rigidity of their structures and thus the absence of the large skeleton conformational effects on their ECD spectra. An additional advantage of the selected enones is the absence of other chromophoric units that could contribute to the overall ECD spectra.
We intend to arrive at the planned goal by the examination of impact of conformation of enone unit on the diagnostic ECD bands. For the purpose of our studies, the band attributed to the enone nπ* transition occurring between 335–360 nm is of a particular interest because this band is the subject of the enone helicity rule. Moreover, in contrast to the band associated with the ππ* enone transition, the long-wavelength CD band is relatively distant from the other, higher energy bands, which could cause some confusing band overlapping. Thus, the analysis of long-wavelength spectral region should simplify the stereochemical correlations.
At the outset, we need to identify in detail the geometry of our model compounds, as the knowledge of all CD-relevant conformers is regarded as a decisive factor for conducting proper chiral analysis. Next, a thorough analysis of the structural parameters along with the ECD data will be carried out through the combined use of electronic circular dichroism spectroscopy and time-dependent density functional theory (TDDFT). Such an approach should enable us to explore and gain new insight into the relationship between stereochemistry and chiroptical properties of examined cis-enones. At the conclusion of this study, we expect to develop a convenient and reliable rule correlating the stereostructure of such enones with their ECD spectra in the nπ* region.
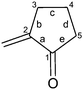
For the purpose of this work we redefined the term “configurational type”. Until now, the term was used by Gawroński for both trans- and cis-enones in the analysis of the structure – sign of the CE's relationship.15 The same term is also known as (i) “absolute configuration of the polycyclic enone” where P-type is defined by the right-handed helicity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C–R bond system (Fig. 1a) and M-type with left-handed helicity of the same system;18 (ii) “the helicity of the Cα = Cβ–Cγ–R torsion angle”19 and (iii) “the absolute configuration at the allylic position”.22 In our analysis of the stereochemistry of cis-enones we use the term “configurational type”, where P-type is defined by the right-handed helicity (positive torsion angle) of the Cβ = Cα–Cε–X bond system, provided that the Cε–X bond is axial or quasi-axial (Fig. 1b). X is an atom of a substituent (H, F, Cl, Br, OR, alkyl, etc.) or atom of an additional ring (e.g. Fig. 1c). Cyclic ketone may be cyclobutanone, cyclopentanone, cyclohexanone, etc., whereas the double bond may be an exocyclic or endocyclic.
C–C–R bond system (Fig. 1a) and M-type with left-handed helicity of the same system;18 (ii) “the helicity of the Cα = Cβ–Cγ–R torsion angle”19 and (iii) “the absolute configuration at the allylic position”.22 In our analysis of the stereochemistry of cis-enones we use the term “configurational type”, where P-type is defined by the right-handed helicity (positive torsion angle) of the Cβ = Cα–Cε–X bond system, provided that the Cε–X bond is axial or quasi-axial (Fig. 1b). X is an atom of a substituent (H, F, Cl, Br, OR, alkyl, etc.) or atom of an additional ring (e.g. Fig. 1c). Cyclic ketone may be cyclobutanone, cyclopentanone, cyclohexanone, etc., whereas the double bond may be an exocyclic or endocyclic.
By analogy to the relation “positive (P) or negative (M) enone helicity” exemplifying “+ or − ω enone torsion angle” we introduce the relation “P or M configurational type” represented by “+ or − σ torsion angle”, where σ refers to value of the Cβ = Cα–Cε–X torsion angle.
2. Experimental and computational details
2.1. Experimental details
All solvents were dried and distilled before use. All reactions were monitored by thin-layer chromatography using aluminum backed silica gel plates 60 F254; visualization was accomplished with UV light and/or staining with 50% H2SO4. Standard flash chromatography procedures were followed (using silica gel with particle size 40–63 μm). Melting points were recorded on a melting point stage and are not corrected. Optical rotation was measured in CH2Cl2 solutions at ambient temperature and is quoted in units of 10−1 deg cm2 g−1. FT-IR spectra were recorded on a FT-IR spectrophotometer for KBr pellets or films. 1H NMR spectra were recorded at 400 or 500 MHz and 13C NMR at 100 or 125 MHz using CDCl3 as solvent and TMS as internal standard and are reported as δ values (ppm) relative to residual CHCl3 signal δH (7.26 ppm) and CDCl3 δC (77.16 ppm) as internal standards, respectively. Electrospray ionization (ESI) mass spectrometry (MS) experiments were performed on a mass spectrometer under normal conditions. PFK solution was used as calibrant for HRMS measurements.UV spectra were measured using acetonitrile as a solvent. CD spectra were recorded between 180–450 nm at room temperature using acetonitrile solutions. Solutions with concentrations in the range 3.5 × 10−4 to 10 × 10−4 mol dm−3 were examined in cells with path length 0.1, 0.2, 0.5 or 1 cm.
3β-Acetoxy-16-methyleneandrost-5-en-17-one (1) was prepared starting from 3β-acetoxyandrost-5-en-17-one (9; dehydroisoandrosterone 3-acetate), according to the known procedure28,29 i.e. treatment of ketones with N,N,N′N′-tetramethyldiaminomethane in hot acetic anhydride (Scheme 1).
To a mixture of 3β-acetoxyandrost-5-en-17-one (9; 1 g) and N,N,N′N′-tetramethyldiaminomethane (10 mL) acetic anhydride (10 mL) was slowly added and the resulting solution was heated at 100–105 °C for 4 h. Then the reaction mixture was acidified with 5% hydrochloric acid and extracted with dichloromethane. The extract was washed with aqueous NaHCO3 solution, dried, filtered and evaporated to dryness. The residue was purified by column chromatography. Crystallization gave enone 1 (0.279 g, 27%), characterized as follows: mp 166.5–167.5 °C (from CH2Cl2–hexane) (lit.: 159–161 °C,30 163–165 °C,31); IR (KBr): 3086, 3038, 1729 (conj. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1640 (conj. C
O), 1640 (conj. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1252, 1033, 1014 and 941 cm−1; δH (400 MHz; CDCl3; Me4Si): 0.92 (3H, s, 18-H), 1.06 (3H, s, 19-H), 2.04 (3H, s, OAc), 2.59 (1H, ddt, J = 15.5, 6.3 and 1.5 Hz, 15α-H), 4.61 (1H, broad m, 3α-H), 5.38 (1H, narrow m,
C), 1252, 1033, 1014 and 941 cm−1; δH (400 MHz; CDCl3; Me4Si): 0.92 (3H, s, 18-H), 1.06 (3H, s, 19-H), 2.04 (3H, s, OAc), 2.59 (1H, ddt, J = 15.5, 6.3 and 1.5 Hz, 15α-H), 4.61 (1H, broad m, 3α-H), 5.38 (1H, narrow m, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHA), 5.41 (1H, broadened d, J = 5.3 Hz, 6-H) and 6.07 (1H, narrow m,
CHA), 5.41 (1H, broadened d, J = 5.3 Hz, 6-H) and 6.07 (1H, narrow m, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHB) [lit.,30 (CDCl3): 0.92 (s, 3H), 1.06 (s, 3H), 2.04 (s, 3H), 4.60 (m, 1H), 5.38 (m, 1H), 5.41 (m, 1H) and 6.07 (m, 1H)]; δC (125 MHz; CDCl3; Me4Si): 13.90, 19.33, 20.29, 21.40, 27.66, 29.27, 30.80, 31.02, 31.39, 36.76, 36.84, 38.05, 47.64, 48.91, 50.10, 73.69, 118.63, 121.76, 139.99, 144.36, 170.51 and 208.59.
CHB) [lit.,30 (CDCl3): 0.92 (s, 3H), 1.06 (s, 3H), 2.04 (s, 3H), 4.60 (m, 1H), 5.38 (m, 1H), 5.41 (m, 1H) and 6.07 (m, 1H)]; δC (125 MHz; CDCl3; Me4Si): 13.90, 19.33, 20.29, 21.40, 27.66, 29.27, 30.80, 31.02, 31.39, 36.76, 36.84, 38.05, 47.64, 48.91, 50.10, 73.69, 118.63, 121.76, 139.99, 144.36, 170.51 and 208.59.
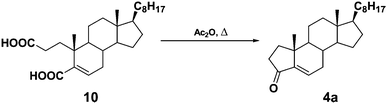
A-norcholest-5-en-3-one (4a) was obtained from cholesterol following the sequence: cholest-5-en-3β-ol (cholesterol) → Diels's acid (10)32 → A-norcholest-5-en-3-one (4a)33 (Scheme 2), and chromatographic purification of the crude product.
The purified enone 4a has been characterized as follows: mp 86–88 °C (from MeOH) (lit.: 95–96 °C,33 99–100 °C (ref. 34)); IR (KBr): 1722 (conj. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1655 (conj. C
O), 1655 (conj. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1230, 1102, 960 and 784 cm−1; δH (500 MHz; CDCl3; Me4Si): 0.72 (3H, s, 18-H), 0.867 (3H, d, J = 6.6 Hz, 27-H), 0.871 (3H, d, J = 6.6 Hz, 26-H), 0.93 (3H, d, J = 6.5 Hz, 21-H), 1.02 (3H, s, 19-H), 1.97 (1H, dd, J = 12.2 and 8.7 Hz, 1β-H), 2.06 (1H, dt, J = 12.7 and 3.4 Hz, 12β-H), 2.25 (1H, dd, J = 18.8 and 8.3 Hz, 2α-H), 2.33 (1H, ddd, J = 20.6, 6.0 and 3.9 Hz, 7β-H), 2.43 (1H, ddd, J = 18.8, 12.1 and 8.8 Hz, 2β-H), 6.56 (1H, t, J ≈ 3.7 Hz, 6-H); δC (125 MHz; CDCl3; Me4Si): 12.01, 18.74, 20.42, 21.78, 22.55, 22.81, 23.81, 24.35, 28.00, 28.13, 31.92, 32.30, 34.75, 35.68, 35.73, 36.15, 39.45, 39.49, 41.48, 42.88, 49.46, 56.06, 56.37, 130.98, 146.55 and 207.38.
C), 1230, 1102, 960 and 784 cm−1; δH (500 MHz; CDCl3; Me4Si): 0.72 (3H, s, 18-H), 0.867 (3H, d, J = 6.6 Hz, 27-H), 0.871 (3H, d, J = 6.6 Hz, 26-H), 0.93 (3H, d, J = 6.5 Hz, 21-H), 1.02 (3H, s, 19-H), 1.97 (1H, dd, J = 12.2 and 8.7 Hz, 1β-H), 2.06 (1H, dt, J = 12.7 and 3.4 Hz, 12β-H), 2.25 (1H, dd, J = 18.8 and 8.3 Hz, 2α-H), 2.33 (1H, ddd, J = 20.6, 6.0 and 3.9 Hz, 7β-H), 2.43 (1H, ddd, J = 18.8, 12.1 and 8.8 Hz, 2β-H), 6.56 (1H, t, J ≈ 3.7 Hz, 6-H); δC (125 MHz; CDCl3; Me4Si): 12.01, 18.74, 20.42, 21.78, 22.55, 22.81, 23.81, 24.35, 28.00, 28.13, 31.92, 32.30, 34.75, 35.68, 35.73, 36.15, 39.45, 39.49, 41.48, 42.88, 49.46, 56.06, 56.37, 130.98, 146.55 and 207.38.
3β-Acetoxy-9-hydroxy-5α-cholest-8(14)-en-15-one (8a) was synthesized from 7-dehydrocholesterol following the sequence: cholestadien-5,7-3β-ol (7-dehydrocholesterol) → 3β-hydroxy-5α-cholesta-8,14-diene35 → 3β-acetoxy-5α-cholesta-8,14-diene (11) → 3β-acetoxy-9-hydroxy-5α-cholest-8(14)-en-15-one (8a)36 (Scheme 3), and chromatographic separation from a mixture of products. The purified enone 8a has been characterized as follows: mp 194–196 °C (from MeOH) (lit.,36 194–195 °C); IR (KBr): 3462 (O–H), 1732 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, acetate), 1686 (conj. C
O, acetate), 1686 (conj. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1616 (conj. C
O), 1616 (conj. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1248, 1235, 1092 and 1035 cm−1 [lit.,36 (CHCl3): 3460, 1730, 1703 and 1630 cm−1]; δH (500 MHz; CDCl3; Me4Si): 0.82 (3H, s, 19-H), 0.864 (3H, d, J = 6.6 Hz, 27-H), 0.866 (3H, d, J = 6.6 Hz, 26-H), 0.97 (3H, s, 18-H), 1.01 (3H, d, J = 6.3 Hz, 21-H), 2.02 (3H, s, OAc), 2.18 (1H, tt, J = 12.7 and 3.5 Hz, 5α-H), 2.40 (1H, dd, J = 18.8 and 7.7 Hz, 16α-H), 3.93 (1H, ddd, J = 14.6, 4.4 and 2.0 Hz, 7β-H) and 4.72 (1H, broad m, 3α-H) [lit.,36 (100 MHz; CDCl3; Me4Si): 0.82 (19-H), 0.97 (18-H), 3.95 (broad d, J = 14 Hz, 7β-H)]; δC (125 MHz; CDCl3; Me4Si): 15.50, 17.28, 19.16, 21.41, 22.52, 22.65, 22.73, 23.46, 26.97, 27.82, 27.97, 28.33, 29.45, 33.60, 33.75, 34.47, 35.13, 35.74, 39.35, 41.31, 42.52, 43.16, 50.37, 72.94, 74.28, 141.59, 148.05, 170.59 and 208.46.
C), 1248, 1235, 1092 and 1035 cm−1 [lit.,36 (CHCl3): 3460, 1730, 1703 and 1630 cm−1]; δH (500 MHz; CDCl3; Me4Si): 0.82 (3H, s, 19-H), 0.864 (3H, d, J = 6.6 Hz, 27-H), 0.866 (3H, d, J = 6.6 Hz, 26-H), 0.97 (3H, s, 18-H), 1.01 (3H, d, J = 6.3 Hz, 21-H), 2.02 (3H, s, OAc), 2.18 (1H, tt, J = 12.7 and 3.5 Hz, 5α-H), 2.40 (1H, dd, J = 18.8 and 7.7 Hz, 16α-H), 3.93 (1H, ddd, J = 14.6, 4.4 and 2.0 Hz, 7β-H) and 4.72 (1H, broad m, 3α-H) [lit.,36 (100 MHz; CDCl3; Me4Si): 0.82 (19-H), 0.97 (18-H), 3.95 (broad d, J = 14 Hz, 7β-H)]; δC (125 MHz; CDCl3; Me4Si): 15.50, 17.28, 19.16, 21.41, 22.52, 22.65, 22.73, 23.46, 26.97, 27.82, 27.97, 28.33, 29.45, 33.60, 33.75, 34.47, 35.13, 35.74, 39.35, 41.31, 42.52, 43.16, 50.37, 72.94, 74.28, 141.59, 148.05, 170.59 and 208.46.
3β-Acetoxy-5α-cholest-8(14)-en-15-one (7a) was synthesized from 7-dehydrocholesterol following the sequence: cholestadien-5,7-3β-ol (7-dehydrocholesterol) → 7-dehydrocholesterol acetate → 3β-acetoxy-5α-cholest-7-ene37 → 3β-acetoxy-5α-cholest-8(14)-ene (12)38 → 3β-acetoxy-5α-cholest-8(14)-en-15-one (7a)39 (Scheme 3), and chromatographic separation from a mixture of products or, alternatively, from the reductive deoxygenation of 8a (ref. 36) (Scheme 3). The purified enone 7a has been characterized as follows: mp 133–135 °C (from MeOH) (lit.,36,39 134–135 °C); IR (film from CHCl3): 1737 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, acetate), 1700 (conj. C
O, acetate), 1700 (conj. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1623 (conj. C
O), 1623 (conj. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1257 and 1030 cm−1 [lit.,36 (CHCl3): 1743, 1705 and 1630 cm−1]; δH (500 MHz; CDCl3; Me4Si): 0.73 (3H, s, 19-H), 0.863 (3H, d, J = 6.6 Hz, 27-H), 0.865 (3H, d, J = 6.6 Hz, 26-H), 0.97 (3H, s, 18-H), 1.00 (3H, d, J = 6.6 Hz, 21-H), 2.03 (3H, s, OAc), 2.07 (1H, dd, J = 18.5 and 6.1 Hz, 16β-H), 2.10 (1H, dt, J = 12.7 and 3.4 Hz, 12β-H), 2.35 (1H, dd, J = 18.5 and 7.8 Hz, 16α-H), 4.13 (1H, ddd, J = 14.3, 4.3 and 2.0 Hz, 7β-H) and 4.73 (1H, broad m, 3α-H) [lit.,36 (100 MHz; CDCl3; Me4Si): 0.98 (18-H), 4.18 (broad d, J = 14 Hz, 7β-H)]; δC (100 MHz; CDCl3; Me4Si): 12.82, 18.78, 19.23, 19.54, 21.40, 22.53, 22.73, 23.53, 27.23, 27.50, 27.97, 29.03, 33.64, 34.51, 35.83, 36.29, 36.94, 38.68, 39.38, 42.45, 42.57, 43.95, 50.75, 50.82, 73.21, 140.44, 150.21, 170.65 and 208.08.
C), 1257 and 1030 cm−1 [lit.,36 (CHCl3): 1743, 1705 and 1630 cm−1]; δH (500 MHz; CDCl3; Me4Si): 0.73 (3H, s, 19-H), 0.863 (3H, d, J = 6.6 Hz, 27-H), 0.865 (3H, d, J = 6.6 Hz, 26-H), 0.97 (3H, s, 18-H), 1.00 (3H, d, J = 6.6 Hz, 21-H), 2.03 (3H, s, OAc), 2.07 (1H, dd, J = 18.5 and 6.1 Hz, 16β-H), 2.10 (1H, dt, J = 12.7 and 3.4 Hz, 12β-H), 2.35 (1H, dd, J = 18.5 and 7.8 Hz, 16α-H), 4.13 (1H, ddd, J = 14.3, 4.3 and 2.0 Hz, 7β-H) and 4.73 (1H, broad m, 3α-H) [lit.,36 (100 MHz; CDCl3; Me4Si): 0.98 (18-H), 4.18 (broad d, J = 14 Hz, 7β-H)]; δC (100 MHz; CDCl3; Me4Si): 12.82, 18.78, 19.23, 19.54, 21.40, 22.53, 22.73, 23.53, 27.23, 27.50, 27.97, 29.03, 33.64, 34.51, 35.83, 36.29, 36.94, 38.68, 39.38, 42.45, 42.57, 43.95, 50.75, 50.82, 73.21, 140.44, 150.21, 170.65 and 208.08.
3β,9-Dihydroxy-5α-cholest-8(14)-en-15-one (8b). Hydrolysis of the acetate 8a with methanol–tetrahydrofuran–aqueous sodium hydroxide gave the alcohol 8b, mp 212–214 °C (from MeOH); [α]D (20 °C) 183.9 (c 1.11 in CH2Cl2); IR (film from CH2Cl2): 3458 (O–H), 3193 (O–H), 1687 (conj. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1623 (conj. C
O), 1623 (conj. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1093 and 1023 cm−1; δH (500 MHz; CDCl3; Me4Si): 0.81 (3H, s, 19-H), 0.864 (3H, d, J = 6.6 Hz, 27-H), 0.866 (3H, d, J = 6.6 Hz, 26-H), 0.97 (3H, s, 18-H), 1.02 (3H, d, J = 6.3 Hz, 21-H), 2.40 (1H, dd, J = 18.8 and 7.7 Hz, 16α-H), 3.64 (1H, broad m, 3α-H) and 3.94 (1H, ddd, J = 14.6, 4.4 and 2.0 Hz, 7β-H); δC (125 MHz; CDCl3, Me4Si): 15.62, 17.28, 19.16, 22.52, 22.72, 23.46, 27.88, 27.97, 28.42, 29.65, 30.89, 33.63, 34.46, 35.28, 35.75, 37.92, 39.34, 41.34, 42.54, 43.16, 50.38, 70.55, 74.41, 141.52, 148.27 and 208.47; ESI HRMS: calcd for [M + Na]+ C27H44O3Na, 439.3188; found, 439.3187.
C), 1093 and 1023 cm−1; δH (500 MHz; CDCl3; Me4Si): 0.81 (3H, s, 19-H), 0.864 (3H, d, J = 6.6 Hz, 27-H), 0.866 (3H, d, J = 6.6 Hz, 26-H), 0.97 (3H, s, 18-H), 1.02 (3H, d, J = 6.3 Hz, 21-H), 2.40 (1H, dd, J = 18.8 and 7.7 Hz, 16α-H), 3.64 (1H, broad m, 3α-H) and 3.94 (1H, ddd, J = 14.6, 4.4 and 2.0 Hz, 7β-H); δC (125 MHz; CDCl3, Me4Si): 15.62, 17.28, 19.16, 22.52, 22.72, 23.46, 27.88, 27.97, 28.42, 29.65, 30.89, 33.63, 34.46, 35.28, 35.75, 37.92, 39.34, 41.34, 42.54, 43.16, 50.38, 70.55, 74.41, 141.52, 148.27 and 208.47; ESI HRMS: calcd for [M + Na]+ C27H44O3Na, 439.3188; found, 439.3187.
3β-Hydroxy-5α-cholest-8(14)-en-15-one (7b). Hydrolysis of the acetate 7a with methanol–tetrahydrofuran–aqueous sodium hydroxide gave the alcohol 7b, mp 146–147 °C (from MeOH) (lit.,36,39 145–146 °C); [α]D (20 °C) 143.3 (c 0.79 in CH2Cl2); IR (film from CH2Cl2): 3406 (O–H), 1703 (conj. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1621 (conj. C
O), 1621 (conj. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1122, 1088 and 1045 cm−1; δH (500 MHz; CDCl3; Me4Si): 0.72 (3H, s, 19-H), 0.86 (6H, broadened d, J = 6.6 Hz, 27-H and 26-H), 0.97 (3H, s, 18-H), 1.00 (3H, d, J = 6.6 Hz, 21-H), 2.35 (1H, dd, J = 18.5 and 7.8 Hz, 16α-H), 3.65 (1H, broad m, 3α-H) and 4.14 (1H, ddd, J = 14.3, 4.1 and 2.0 Hz, 7β-H); δC (125 MHz; CDCl3; Me4Si): 12.93, 18.79, 19.23, 19.59, 22.53, 22.74, 23.52, 27.58, 27.97, 29.15, 31.18, 34.50, 35.83, 36.54, 36.98, 37.81, 38.74, 39.37, 42.48, 42.57, 44.14, 50.81, 50.87, 70.95, 140.32, 150.57 and 208.12; ESI HRMS: calcd for [M + Na]+ C27H44O2Na, 423.3239; found, 423.3238.
C), 1122, 1088 and 1045 cm−1; δH (500 MHz; CDCl3; Me4Si): 0.72 (3H, s, 19-H), 0.86 (6H, broadened d, J = 6.6 Hz, 27-H and 26-H), 0.97 (3H, s, 18-H), 1.00 (3H, d, J = 6.6 Hz, 21-H), 2.35 (1H, dd, J = 18.5 and 7.8 Hz, 16α-H), 3.65 (1H, broad m, 3α-H) and 4.14 (1H, ddd, J = 14.3, 4.1 and 2.0 Hz, 7β-H); δC (125 MHz; CDCl3; Me4Si): 12.93, 18.79, 19.23, 19.59, 22.53, 22.74, 23.52, 27.58, 27.97, 29.15, 31.18, 34.50, 35.83, 36.54, 36.98, 37.81, 38.74, 39.37, 42.48, 42.57, 44.14, 50.81, 50.87, 70.95, 140.32, 150.57 and 208.12; ESI HRMS: calcd for [M + Na]+ C27H44O2Na, 423.3239; found, 423.3238.
2.2. Computational details
To rationalize the experimental observations and to extract the meaningful information about enone structure – ECD spectra relationship, the calculations were performed to assist with the interpretation of experimental results. Moreover, the structural factors that potentially could affect the ECD spectra such as ω enone [O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C
C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C] and τ ene torsion angles as well as the conformation of cyclopentanone ring were also analyzed. The τ ene torsion angle is defined as C/H–C
C] and τ ene torsion angles as well as the conformation of cyclopentanone ring were also analyzed. The τ ene torsion angle is defined as C/H–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C(
C–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), where C/H is syn to C–C(
O), where C/H is syn to C–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) bond.22,40 A fundamental prerequisite for the computational calculation of ECD spectra is the knowledge of all conformational species of the respective molecule, therefore our studies started with conformational analysis at the molecular mechanics level employing the MM2 force field.41 In order to simplify computation and to save the CPU time and costs, the C8H17 side chain at C(17) in enones 4a, 7a and 8a was replaced by the place-holder methyl group. Both groups, i.e. C8H17 and CH3, have very similar electronic properties, and interchanging them does not change the electronic spectra significantly. Therefore, the calculations were performed for the structurally simplified enones 4b, 7c and 8c. The basic data are summarized in Table 1, whereas the complete results of conformational analysis for compounds 1–3, 4b, 5, 6, 7c and 8c as well as compounds 15–18 are collected and presented in ESI.† For all compounds studied, with the exception of compound 18, the major conformers used to the simulation of Boltzmann-averaged ECD spectra showed very similar values for the crucial torsional angles of 2-methylenecyclopentanone unit (σ, ω, τ and “a–e”; see Table S5†). Only in the case of enone 18, its two lowest-energy conformers differed significantly in torsion angles σ, ω, τ, “a” and “b”.
O) bond.22,40 A fundamental prerequisite for the computational calculation of ECD spectra is the knowledge of all conformational species of the respective molecule, therefore our studies started with conformational analysis at the molecular mechanics level employing the MM2 force field.41 In order to simplify computation and to save the CPU time and costs, the C8H17 side chain at C(17) in enones 4a, 7a and 8a was replaced by the place-holder methyl group. Both groups, i.e. C8H17 and CH3, have very similar electronic properties, and interchanging them does not change the electronic spectra significantly. Therefore, the calculations were performed for the structurally simplified enones 4b, 7c and 8c. The basic data are summarized in Table 1, whereas the complete results of conformational analysis for compounds 1–3, 4b, 5, 6, 7c and 8c as well as compounds 15–18 are collected and presented in ESI.† For all compounds studied, with the exception of compound 18, the major conformers used to the simulation of Boltzmann-averaged ECD spectra showed very similar values for the crucial torsional angles of 2-methylenecyclopentanone unit (σ, ω, τ and “a–e”; see Table S5†). Only in the case of enone 18, its two lowest-energy conformers differed significantly in torsion angles σ, ω, τ, “a” and “b”.
Conformers found by the conformational analysis were subjected to the quantum chemical geometry optimization using B3LYP density functional and TZVP basis set as implemented in Gaussian09 program package.42 Since the ECD experiments were conducted in a solution, the structure optimization by DFT methods was evaluated for the solvation effect using the polarizable continuum model (PCM).43 Rotatory strengths were calculated using both length and velocity representations. The differences between the length and velocity of the calculated values of the rotatory strengths were <5%, and for this reason, only the velocity representations (Rvel) were taken into account. The theoretical ECD spectra were simulated using B3LYP functional and TZVP basis set with PCM model for acetonitrile or methanol (for enone 2) as a solvent. When necessary, the calculated spectra were wavelength corrected to match the experimental UV maxima. Throughout the paper the value of this UV correction factor is given in the respective figures.
It should be noted that the use of other density functionals such as CAM-B3LYP, PBE0, LC-wPBE, M06-2X, 23LYP and B2LYP as well as CC2 method led to either similar or actually even less accurate results than those obtained with the use of B3LYP hybrid functional (these data are deposited in the additional ESI† and are available on request from the authors).
3. Results and discussion
3.1. Conformational analysis of enones 1–8
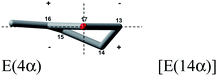
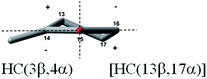
Conformational analysis showed that enones 1–8 can be divided into two distinct groups depending on the value of enone torsion angle (ω). The first group consists of enones 1 and 2 with a nearly planar chromophore as shown by their enone torsion angles (Table 1), very similar to those of bornan-3-one derivatives (−4°) classified as planar cis-enones.18 The second group includes the non-planar enones 3–8 with enone torsion angles higher than −/+10° (Table 1).The conformation of cyclopentanone ring is described by the torsion angles “a–e”, defined in figure included in Table 1. The unified numbering of the cyclopentanone ring is given to identify the conformation of this ring. In the octant-like projection of the cyclopentanone ring all enones are viewed along the axis of the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond with C
C bond with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond on the left hand side of the cyclopentanone ring. As can be seen in Table 1, the cyclopentanone ring of investigated enones adopts conformation close to an envelope (E) when either the carbon atom 4 or carbon atom 3 is positioned outside the plane of the carbonyl group or a conformation close to a half-chair (HC) when both these carbon atoms are positioned outside the same plane.
C bond on the left hand side of the cyclopentanone ring. As can be seen in Table 1, the cyclopentanone ring of investigated enones adopts conformation close to an envelope (E) when either the carbon atom 4 or carbon atom 3 is positioned outside the plane of the carbonyl group or a conformation close to a half-chair (HC) when both these carbon atoms are positioned outside the same plane.
It is worth to emphasize that for all conformers of examined enones the differences in enone torsion angle and in cyclopentanone torsion angles “a–e” are small (see ESI†). This is a consequence of the rigidity of their molecular skeletons. Finally, a very good agreement seen for a direct comparison of the geometry-related data obtained from a single crystal X-ray diffraction analysis of 8a and data obtained from the molecular modeling of its analog 8c validates the correctness of chosen calculation methodology (Table 1, Fig. 2).
 | ||
| Fig. 2 ORTEP diagram of compound 8a with displacement ellipsoids drawn at the 50% probability level (left) and conformation of the lowest energy conformer of enone 8c (right). | ||
3.2. Experimental ECD results of enones 1–8
The UV and ECD spectra of enones 1 and 3–8b (Chart 1) were recorded in acetonitrile and the collected data are presented in Table 2. The ECD data for enone 2 recorded in methanol are taken from the literature.44 As evident from Table 2, up to four absorption bands within the spectral range 200–360 nm are present in the ECD spectra of investigated compounds. The long-wavelength band occurring at around 350 nm is attributed to the enone nπ* transition whereas the one arising in the range ∼230 to 270 nm is assigned to the ππ* excitation of the same chromophore unit.| enone | UV ε (λmax); [M−1 cm−1 (nm)] | CD Δε (λmax); [M−1 cm−1 (nm)] | ||||
|---|---|---|---|---|---|---|
| a Data taken from literature.44b 4a probably exhibits a bisignate ππ* CE.15c Commercially available. | ||||||
| 1 | 8900 (226) | 70 (348) | −11.6 (228) | +1.7 (352) | ||
| 2a | 8800 (243) | 77 (345) | −1.1 (212) | +1.5 (238) | −3.9 (341) | |
| 3 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 (240) 800 (240) |
150 (348) | +0.7 (213) | +0.7 (260) | −3.8 (350) | |
| 4ab | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (242) 100 (242) |
45 (338) | −6.4 (226) | +0.2 (270) | −1.4 (336) | |
| 5 | 8550 (240) | 30 (345) | +2.0 (206) | −15.7 (240) | −1.2 (345) | |
| 6c | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (252) 100 (252) |
61 (357) | +2.4 (199) | −1.9 (215) | −6.8 (252) | −1.1 (356) |
| 7a | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 (257) 560 (257) |
79 (349) | −1.7 (201) | +3.9 (227) | +3.1 (255) | +1.9 (347) |
| 7b | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 (258) 050 (258) |
80 (349) | −1.2 (201) | +3.8 (227) | +3.8 (256) | +1.8 (347) |
| 8a | 8950 (253) | 51 (354) | −1.2 (205) | +7.5 (251) | +1.2 (350) | |
| 8b | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 (253) 300 (253) |
78 (353) | −1.1 (205) | +12.0 (252) | +1.7 (350) | |
The experimental ECD spectrum of cis-enone 1 shows two bands within the range 360–210 nm. A positive band found at 352 nm corresponds to the nπ* transition and a negative band at 228 nm corresponds to the ππ* transition. The ECD spectra of compounds 2–6 within the same spectral range exhibit a negative band for nπ* transition at 341, 350, 336, 345 and 356 nm, respectively. However, they differ in the signs of bands below 300 nm. The band corresponding to the ππ* transition is positive in the case of enones 2, 3 and 4a (at 238, 260 and 270 nm, respectively) but negative for enones 5 and 6 (at 240 and 252 nm, respectively). Moreover, for compounds 2, 3, 4a and 6 a third band in the range 230–210 nm is observed. Its sign is opposite to the sign of the ππ* band for compounds 2 and 4a and is the same for enones 3 and 6. Cis-enones 7a, 7b, 8a and 8b display two bands in the range 360–240 nm. Positive bands corresponding to the nπ* transition are present at around 350 nm and the bands of the same sign corresponding to the ππ* transition are found at about 255 nm. Furthermore, compounds 7a and 7b show an additional positive band at 227 nm.
An examination of data from Table 2 additionally shows that the investigated enones can be divided into two distinct groups depending on the sign of the CE's for nπ* and ππ* transitions. The first group, consisting of enones 1–4, displays the opposite signs of these two transitions while the second group, composed of enones 5–8b, shows the same sign for both CE's attributed to these transitions.
3.3. Theoretical ECD results and their comparison with experimental data for enones 1–8
The simulated Boltzmann-averaged ECD spectra of planar cis-enones 1 and 2 are in a very good agreement with the experimental ECD data for diagnostic bands corresponding to the nπ* and ππ* transitions. The calculations confirm a presence of a positive CE at 352 nm and a negative one at 228 nm for enone 1 (Fig. 3 left) as well as a negative CE at 341 nm and a positive one at 238 nm for enone 2 (Fig. 3 middle). The cyclopentanone ring of compound 1 adopts conformation similar to an envelope E(4α) and its octant-like projection (Fig. 3 left) shows a positive contribution to the nπ* transition. For compound 2, the octant-like projection of the ketone ring, existing in the conformation similar to the envelope E(4β), exhibits a negative contribution to the nπ* CE (Fig. 3 middle). Thus, the sign of the nπ* CE of enones 1 and 2 can be correlated with the chirality of cyclopentanone ring. In both cases, the sign of the ππ* CE is opposite to that of nπ* CE.Enones 3, 4a, 5 and 6 with positive enone torsion angles show the negative nπ* CE's (Table 2). For these compounds the shape of the experimental ECD curves agrees well with the calculated curves (Fig. 3 right and 4). Conformational analysis shows that the cyclopentanone ring of compounds 3 and 4b adopts a similar conformation, close to a half-chair HC(3α,4β) (Fig. 3 right and 4 left). Their octant-like projections suggest a negative contribution to the nπ* transition and expectedly this results in the negative nπ* CE. Thus, similarly to the planar enones 1 and 2, the chirality of cyclopentanone ring is responsible for the correlation of molecular structure and relevant ECD data. In addition, calculations done for the enone 4b show that very weak positive band at 270 nm corresponds to the ππ* transition of the enone chromophore. This result contradicts the earlier suggestion that compound 4a exhibits a bisignate ππ* CE.15,45 Therefore, in the case of compounds 3 and 4a, the sign of the ππ* CE is opposite to that of nπ* CE.
The conformational analysis of cis-enones 5 and 6 shows that their cyclopentanone rings adopt conformation close to an envelope E(3α) or E(4β), respectively (Fig. 4 middle and right). Their octant-like projections display a negative contribution to the nπ* transition. Thus, the negative sign of the nπ* CE of compounds 5 and 6 can be correlated with the chirality of cyclopentanone ring. However, in both cases (compounds 5 and 6) the observed and calculated nπ* and ππ* CE's have the same negative sign.
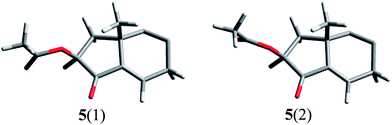
Moreover, the calculations for two conformers of enone 5 showed that even a small difference in conformation of acetoxyl substituent at α′ position to the ketone carbonyl group (Fig. 5) leads to a large difference between rotatory strength values for the nπ* transition [−6.5 × 10−40 cgs units for 5(1) and −0.5 × 10−40 cgs units for 5(2)].
Enones 7a, 7b, 8a and 8b have the same Δ8(14)-15-oxo enone moiety but they differ in respect to the substitution at C(9) position – 9α-H for 7a and 7b and 9α-OH for 8a and 8b. Another difference is the identity of substituent at the C(3) position – 3β-OAc for 7a and 8a and 3β-OH for 7b and 8b. All these compounds should have negative enone torsion angles, as found for their simplified analogs 7c and 8c from the computational study. This assumption is adequately confirmed by the result of single crystal X-ray diffraction analysis of 8a (see Table 1). Accordingly, an agreement between the experimental ECD spectrum for 7a and the Boltzmann-averaged ECD spectrum for 7c is very satisfactory (Fig. 6, left). The same is valid for the experimental ECD spectrum of enone 8a and the Boltzmann-averaged ECD spectrum of enone 8c (Fig. 6, right). As expected, the replacement of 3β-acetoxy substituent in 7a and 8a by the 3β-hydroxyl in 7b and 8b did not introduce any visible change in their ECD spectra (Table 2). As can be seen at Fig. 6, the cyclopentanone ring of compounds 7c and 8c adopts practically the same conformation [close to a half-chair HC(3β,4α)] and the positive sign associated with sectors occupied by carbon atoms of cyclopentanone ring corresponds to the observed positive nπ* CE's of enones 7a, 7b, 8a and 8b. Analogously to compounds 5 and 6, the nπ* and ππ* CE's of enones 7 and 8 both have the same sign, although it is positive now, in the experimental as well as the calculated spectra.
In addition, in the case of enone 8c conformers, a strong influence of the conformation of hydroxyl substituent at γ allylic position (Fig. 7) on the rotatory strength values calculated for ππ* transition is clearly visible. Conformers with the torsion angle H–O–C(9)–C(8) of about −63° show maximal positive rotatory strength values of about +65 × 10−40 cgs units, those with torsion angle of about +51° show medium positive rotatory strength values of about +36 × 10−40 cgs units, whereas the conformers with torsion angle of about −169° show a very low positive rotatory strength values of about +2 × 10−40 cgs units. Moreover, the data presented in Fig. 7 indicate that the contribution of hydroxyl substituent at γ-transoid position can be positive in agreement with the Beecham's postulate46 [conformers 8c(1)–8c(4)] or negative in contradiction to the Beecham's postulate [conformers 8c(5) and 8c(6)], despite the same positive O–C(9)–C(8)=C(14) torsion angle. The difference probably results from the presence or absence of a “Z” arrangement of lone pair–O–C(9)–C(8) fragment, respectively, found in these conformers. To the best of our knowledge, this is probably the first observation of such dualistic behavior of substituent in ECD spectra. The observed influence of 9α-hydroxyl substituent on rotatory strength values of the nπ* transition is much smaller.
 | ||
| Fig. 7 Fragments of conformers of compound 8c and of reference conformers of compound 7c. R is given in 10−40 cgs units. | ||
From the analysis of data discussed above (see Table 3) it was established that (i) the sign of the nπ* CE of planar cis-enones 1 and 2 can be predicted utilizing sector rule with the sign pattern applicable to the saturated ketones (octant-like rule); (ii) the sign of the nπ* CE of skewed cis-enones 3–8b can be predicted by application of the same octant-like rule as for planar compounds; (iii) the sign of the nπ* CE of skewed enones 3–8b follows the first enone helicity rule since it is opposite to the sign of the enone torsion angle; (iv) the common feature of all investigated cis-enones is the correlation of sign of the nπ* CE's with their M or P configurational type,15,18,19,22 i.e. P configurational type predicts a positive nπ* CE and M configurational type anticipates a negative nπ* CE.
| Enone | Observed and calcd nπ* CE | nπ* CE from octant-like rule | Enone torsion angle ω | nπ* CE from the EHR | nπ* CE from the EOHR | ct | nπ* CE from ct |
|---|---|---|---|---|---|---|---|
| 1 | + | + | ∼0 (small +) | No | No | P | + |
| 2 | − | − | ∼0 (small +) | No | No | M | − |
| 3 | − | − | + | − | + | M | − |
| 4a,b | − | − | + | − | + | M | − |
| 5 | − | − | + | − | + | M | − |
| 6 | − | − | + | − | + | M | − |
| 7a,b,c | + | + | − | + | − | P | + |
| 8a,b,c | + | + | − | + | − | P | + |
In the light of the above, the question arises which specific factors are responsible for the sign of the nπ* CE? To clarify this issue, we decided to carry out the detailed calculations of the relationship between the geometry and the chiroptical properties for a set of additional model enones. Our expectation was that the outcome of these calculations should at least allow approximating these correlations, if not explain them entirely. Above all, we wanted to find the main factor responsible for the structure – chiroptical properties relationship.
3.4. Conformational analysis and theoretical ECD results for s-cis acrolein (13), 2-methylenecyclopentanone (14) and model cis-enones 15–18
The first objective was to determine the contributions from both the twisted ene (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and from the twisted enone (O
C) and from the twisted enone (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C
C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) relevant to the rotatory strength, i.e. a dependence of the rotatory strength on the torsion angles τ (defined in point 2.2) and ω. This problem was not investigated earlier for cis-enones at the appropriate level of theory. As models for these studies s-cis acrolein (13) and 2-methylenecyclopentanone (14) were selected (Chart 2).
C) relevant to the rotatory strength, i.e. a dependence of the rotatory strength on the torsion angles τ (defined in point 2.2) and ω. This problem was not investigated earlier for cis-enones at the appropriate level of theory. As models for these studies s-cis acrolein (13) and 2-methylenecyclopentanone (14) were selected (Chart 2).
A further objective was to determine which structural factors (nonplanarity of the chromophore, cyclopentanone ring conformations or other elements) are primarily responsible for the sign of the nπ* rotatory strength. We expected that the answer should be found from the analysis of data obtained for our model cis-enones 1–8, 2-methylenecyclopentanone (14) as well as other appropriately selected polycyclic cis-enones.
Since conformations close to an envelope as well as a half-chair were found for cis-enones derived from all-trans condensed policyclic skeletons, we decided to extend our study to conformation of model enones with cis-fused arrays of rings or with the cyclopentanone ring located inside a polycyclic skeleton. For this purpose, four additional model cis-enones 15, 16, 17 and 18 presented in Chart 2 (namely 17β-methyl-B-norandrost-4-en-6-one, 17β-methyl-B-nor-8α-androst-4-en-6-one, 17β-methyl-B-nor-5α,9β-androst-8(14)-en-6-one and 17β-methyl-B-nor-5β,9β-androst-8(14)-en-6-one, respectively) were selected and computationally analyzed.
s-cis-Acrolein (13) represents the simplest model of cis-enone. For the purpose of our analysis, the torsion angle ω was varied in the range from −25° to +25°, in five-degree steps. The torsion angle τ was varied in the range between −5° to +5°, in one-degree steps. For all conformers, the rotatory strength of the nπ* transition was calculated at B3LYP/aug-cc-pVDZ/PCM (acetonitrile) level. The results are shown in Table S1 (ESI†) and visualized on Fig. 8 and S1 (ESI†).
Fig. 8 shows rotatory strength (R) as a function of torsion angle ω (left graph; the cases where C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is planar [τ = 0°]) and as a function of torsion angle τ (middle graph; the cases where enone unit is planar [ω = 0°]). For conformers with a planar C
C bond is planar [τ = 0°]) and as a function of torsion angle τ (middle graph; the cases where enone unit is planar [ω = 0°]). For conformers with a planar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (torsion angle τ = 0°) a positive ω angle results in a negative nπ* rotatory strength and vice versa thus being in a compliance with the first enone helicity rule. It should be noted that the calculated rotatory strength for this transition is not a simple function of the torsion angle ω (Fig. 8, and Table S1†). The deviation of the C
C bond (torsion angle τ = 0°) a positive ω angle results in a negative nπ* rotatory strength and vice versa thus being in a compliance with the first enone helicity rule. It should be noted that the calculated rotatory strength for this transition is not a simple function of the torsion angle ω (Fig. 8, and Table S1†). The deviation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond from planarity generates also the difference in calculated rotatory strength. For all conformers with planar enone (torsion angle ω = 0°), the negative torsion angle τ generates a positive rotatory strength and the positive torsion angle generates a negative rotatory strength. Thus, the dependence of the sign of rotatory strength of nπ* transition on the sign of the angle τ is the same as the dependence on the sign of the angle ω. The maximal rotatory strength generated by the deviation from planarity of the C
C bond from planarity generates also the difference in calculated rotatory strength. For all conformers with planar enone (torsion angle ω = 0°), the negative torsion angle τ generates a positive rotatory strength and the positive torsion angle generates a negative rotatory strength. Thus, the dependence of the sign of rotatory strength of nπ* transition on the sign of the angle τ is the same as the dependence on the sign of the angle ω. The maximal rotatory strength generated by the deviation from planarity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond has a lower intensity compared to that calculated for a twisted enone, while for ω = 0° is equal to nearly one fourth of the latter. Therefore, for a positive torsion angle ω lower than 2° and for a negative torsion angle τ in the range −1° to −5° the calculated values of the rotatory strength are positive, whereas for a negative torsion angle ω lower than −2° and a positive torsion angles τ in the range +1° to +5° the calculated values of the rotatory strength are negative. The contribution to the rotatory strength due to the non-planarity of the C
C bond has a lower intensity compared to that calculated for a twisted enone, while for ω = 0° is equal to nearly one fourth of the latter. Therefore, for a positive torsion angle ω lower than 2° and for a negative torsion angle τ in the range −1° to −5° the calculated values of the rotatory strength are positive, whereas for a negative torsion angle ω lower than −2° and a positive torsion angles τ in the range +1° to +5° the calculated values of the rotatory strength are negative. The contribution to the rotatory strength due to the non-planarity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, calculated as ΔR = Rτ+5° − Rτ−5°, is the highest and negative (ca. −0.30 × 10−40 cgs units) for torsion angle ω 0° and then becomes close to 0 for torsion angles ω higher than ±25°, respectively (Fig. 8, right graph; Table S1†). It means that in general, more positive angle τ generates more negative rotatory strength or alternatively, more negative angle τ generates more positive rotatory strength.
C bond, calculated as ΔR = Rτ+5° − Rτ−5°, is the highest and negative (ca. −0.30 × 10−40 cgs units) for torsion angle ω 0° and then becomes close to 0 for torsion angles ω higher than ±25°, respectively (Fig. 8, right graph; Table S1†). It means that in general, more positive angle τ generates more negative rotatory strength or alternatively, more negative angle τ generates more positive rotatory strength.
A similar analysis was made for the 2-methylenecyclopentanone (14). Conformational analysis identified two lowest-energy enantiomeric conformers called later P-helical conformer and M-helical conformer or shortly P-conformer and M-conformer, whose enone torsion angles ω are +5.2° and −5.2°, respectively. Their ene torsion angles τ are −1.5° and +1.5°, respectively. For the purpose of our analysis, a torsion angle ω was varied in the range −25° to +25°, in five-degree steps. A torsion angle τ, defined as above, was varied in the range −5° to +5°, in one-degree steps. For all conformers the rotatory strength of the nπ* transition was calculated at B3LYP/aug-cc-pVDZ/PCM (acetonitrile) level. Thus, two sets of enantiomeric conformers were obtained. The results are shown in Tables S2 and S3 (ESI†) and visualized at Fig. 9 as well as Fig S2 and S3 (ESI†). Trends of the rotatory strength changes for two sets of enantiomeric conformers of 2-methylenecyclopentanone (14) (Fig. 9) depending on the increasing values of the torsion angle ω and increasing values of the torsion angle τ are similar to those of s-cis acrolein (13). However, for τ = 0°, going from the torsion angle ω −25° to +25°, the calculated positive value of the rotatory strength for P-conformers decreases to the value 0 for ω about +22°, and then becomes negative. The calculated positive value of the rotatory strength for M-conformers at ω −25° decreases to the value 0 for ω about −22°, and then increases in negative value up to ω +25°. Similarly to the s-cis acrolein (13), the maximal rotatory strength generated by the deviation from planarity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond has a lower intensity compared to that calculated for twisted enone, and for ω ca. +5° (P-conformers) and ca. −5° (M-conformers) is equal to nearly one fifth of the latter. In the case of P-conformers the contribution to the rotatory strength due to the nonplanarity of the C
C bond has a lower intensity compared to that calculated for twisted enone, and for ω ca. +5° (P-conformers) and ca. −5° (M-conformers) is equal to nearly one fifth of the latter. In the case of P-conformers the contribution to the rotatory strength due to the nonplanarity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, calculated as ΔR = Rτ+5° − Rτ−5°, is positive for the negative torsion angles ω higher than about −18° and negative for the torsion angles from about −18° to +20°, being highest negative for torsion angle ω of about +5° (ca. −0.33 × 10−40 cgs units) (Fig. 9, right top graph; Table S2†). For M-conformers the contribution to the rotatory strength due to the nonplanarity of the C
C bond, calculated as ΔR = Rτ+5° − Rτ−5°, is positive for the negative torsion angles ω higher than about −18° and negative for the torsion angles from about −18° to +20°, being highest negative for torsion angle ω of about +5° (ca. −0.33 × 10−40 cgs units) (Fig. 9, right top graph; Table S2†). For M-conformers the contribution to the rotatory strength due to the nonplanarity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, calculated in the same manner, is negative for the torsion angles ω from −20° to about +18°, being highest negative for the torsion angle of about −5° (ca. −0.33 × 10−40 cgs units), and positive for the torsion angles higher than about +18° (Fig. 9, right bottom graph; Table S3†). Thus, the nonplanarity of the C
C bond, calculated in the same manner, is negative for the torsion angles ω from −20° to about +18°, being highest negative for the torsion angle of about −5° (ca. −0.33 × 10−40 cgs units), and positive for the torsion angles higher than about +18° (Fig. 9, right bottom graph; Table S3†). Thus, the nonplanarity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond has less importance compared to the distortion of the conjugated chromophore.
C bond has less importance compared to the distortion of the conjugated chromophore.
The most essential conclusion from the above analysis is that for both the negative and the positive torsion angles ω, the calculated values of rotatory strength can be either positive or negative (Fig. 9, left panel). This directly questions the applicability of the first enone helicity rule for the prediction of sign for the nπ* CE's of examined cis-enones. A second important observation originates from the analysis of conformers of 2-methylenecyclopentanone 14. Specifically, the additional conformers, other than encountered earlier, may exist [such as e.g. close to E(5β) or E(5α)], for a sufficiently high positive or high negative values of the torsion angles ω (see Table 4).
Finally, we compared (see Table 5, below) the configurational type of enone (ct), the values of torsion angle designating configurational type of enone (σ), the enone torsion angle (ω), the signs of torsion angles “a–e”, and the calculated values of rotatory strength (Rvel) for the most significant conformers of both series. For P-helical conformers it was found that (i) the starting sequence of signs [−,+,−,+,−] of the torsion angles “a–e” for conformer with ω −25° and τ 0° undergoes the change to sequence [+,+,−,+,−] when the angle ω reaches a value of about +5°; (ii) the determination of configurational type (ct) of enone becomes impossible for conformers with torsion angle ω above ∼+15°; and (iii) for torsion angles higher than +22° the change of sign of rotatory strength (R) may be possible. Analogous conclusions can be drawn for M-helical conformers. In the latter case, the torsion angles (σ, ω, “a–e”) and rotatory strength (Rvel) have the same values, but they are of opposite sign.
| P-helical conformers | M-helical conformers | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nπ* Rvel | ct | Torsion angles [°] | ct | Torsion angles [°] | nπ* Rvel | ||||||||||||||
| σ | ω | τ | a | b | c | d | e | σ | ω | τ | a | b | c | d | e | ||||
| +5.9 | P | +99.9 | −25 | 0 | − | + | − | + | − | M | −99.9 | +25 | 0 | + | − | + | − | + | −5.9 |
| +5.5 | P | +93.1 | −15 | 0 | − | + | − | + | − | M | −93.1 | +15 | 0 | + | − | + | − | + | −5.5 |
| +3.7 | P | +82.5 | 0 | 0 | − | + | − | + | − | M | −82.5 | 0 | 0 | + | − | + | − | + | −3.7 |
| Sign change of the torsion angle “a” | |||||||||||||||||||
| +2.9 | P | +78.8 | +5 | 0 | + | + | − | + | − | M | −78.8 | −5 | 0 | − | − | + | − | + | −2.9 |
| +2.0 | P | +74.9 | +10 | 0 | + | + | − | + | − | M | −74.9 | −10 | 0 | − | − | + | − | + | −2.0 |
| +1.1 | P | +71.1 | +15 | 0 | + | + | − | + | − | M | −71.1 | −15 | 0 | − | − | + | − | + | −1.1 |
| Configurational type (ct) becomes indeterminate | |||||||||||||||||||
| +0.3 | ? | +67.2 or −51.7 | +20 | 0 | + | + | − | + | − | ? | −67.2 or +51.7 | −20 | 0 | − | − | + | − | + | −0.3 |
| Predicted sign change of the rotatory strength (R) | |||||||||||||||||||
| −0.5 | ? | +63.3 or −55.6 | +25 | 0 | + | + | − | + | − | ? | −63.3 or +55.6 | −25 | 0 | − | − | + | − | + | +0.5 |
Continuing our calculations, we searched for cis-enones, which would have conformation of the cyclopentanone ring different than those already found for examined earlier enones 1–8b, i.e. other than E(3α), E(3β), E(4α), E(4β), HC(3α,4β) and HC(3β,4α).
Calculations performed for enone 15 revealed only one conformer 15(1) which shows a positive nπ* CE and a negative ππ* CE (Fig. 10, left). As can be seen (Fig. 10, left), the cyclopentanone ring of compound 15 adopts conformation close to an envelope E(4α) and the positive signs associated with sectors occupied by carbon atoms C(9) and C(10) of the steroid skeleton agree with the calculated positive nπ* CE. A positive nπ* CE can also be predicted by the first enone helicity rule (negative enone torsion angle, ω −15.6°) and by the configurational type of enone (P configurational type, σ +100.8°).
In the case of enone 16 one strongly predominant conformer 16(1) with a very high negative enone torsion angle equal to −37.6° was found (see ESI†). Calculations show positive signs for both nπ* and ππ* CE's (Fig. 10, middle). The cyclopentanone ring adopts conformation close to an envelope E(5α). Such a conformation was not present in the analyzed earlier set of enones. Assuming its octant-like projection (Fig. 10, middle), a weak negative nπ* CE for such conformer would be expected. Thus, the octant-like projection does not allow to predict correctly the sign of nπ* CE for conformer 16(1). However, the positive nπ* CE for this conformer can be predicted by the first enone helicity rule (negative enone torsion angle) and by configurational type of enone (P configurational type, σ +83.1°).
For compound 17 only one significant conformer 17(1) was found from the conformational analysis. It has a strong positive ω torsion angle (+32.2°) and a strong negative τ torsion angle (−10.3°). Calculations done for this enone display a positive nπ* and a positive ππ* CE (Fig. 10, right). As can be seen (Fig. 10, right), the cyclopentanone ring of compound 17 adopts conformation close to an envelope E(5β) and its octant-like projection indicates a weak positive nπ* CE. Such a conformation was also not encountered in the analyzed earlier set of enones. For this enone, both its M configurational type (σ −75.7°) and the first enone helicity rule (positive enone torsion angle, ω +32.2°) do not correctly predict the calculated positive nπ* CE.
The next cis-enone examined computationally was compound 18. For this compound two conformers 18(1) and 18(2) with a positive ω torsion angle (+12.0° and +5.8°, respectively) were found. However, these conformers unexpectedly differ in regard to the sign of τ torsion angle, i.e.; they show negative sign (−4.0°) and positive sign (+9.6°), respectively. Calculations reveal a negative nπ* and a positive ππ* CE for conformer 18(1) and both negative CE's for conformer 18(2) (Fig. 11). The cyclopentanone ring of both conformers adopts slightly different conformation, close to an envelope E(4β). For both conformers the octant-like projections correctly predict the negative nπ* CE's. In this case also the M configurational type (both ct negative) and first enone helicity rule (both positive enone torsion angles) correctly correlate with the calculated negative nπ* CE's. Furthermore, the calculations done for two conformers of enone 18, show a considerable difference between enone torsion angle ω and indicate τ torsion angles of opposite signs which demonstrate a significant dissimilarity between rotatory strength values for both nπ* and ππ* transitions. In this case, substantial changes of angles ω and τ result in significant changes in the value of the rotatory strength, which were not observed for other enones with small changes of angles ω and τ.
3.5. Comprehensive analysis of the results for investigated cis-enones
A comparison of conformations of 2-methylenecyclopentanone unit in all important conformers found for enones 1–8c as well as for theoretical models 15–18 is shown in Table S4.† Additionally, the selected torsion angles data for 2-methylenecyclopentanone units of enones 1–8c and 15–18 are presented in Tables S5 and S6† (for all conformers and for some conformers, respectively). Within these tables, the conformers are pre-sorted, considering as the most important parameter the sign of the calculated rotatory strength (R) for the nπ* transition and the sign and value of the torsion angles ω. The data presented in Tables S5 and S6† clearly show that the sign of torsion angle “b” defines the conformer assignment to the P-helical or M-helical conformer series of 2-methylenecyclopentanone, where the term “P” was adopted arbitrarily for conformers with a positive sign of the torsion angle “b”.Analysis of the data presented in Tables S4, S5 and S6 (ESI†) indicates that the octant-type relation definitively cannot be applied with confidence to correlate the molecular structure and the sign of the nπ* CE because for the enone 16 it leads to a wrong result. An application of the first enone helicity rule is also questionable, since its utilization for the enone 17 with strongly twisted enone chromophore (the torsion angle ω +32.2°) does not provide the correct result. The accurate outcome cannot be obtained upon application of the same rule to compound 1, although it works well for compound 2 (both enones have the conformers with only slightly twisted chromophore; the torsion angles ω between +2.9° and +4.3°). Also, a correlation of the sign of the nπ* CE with the configurational type of enone cannot be taken into account in view of the uncertainty of its determination in some cases (see Table 5).
Further analysis of the results presented in Tables S4–S6† shows that considering the different torsion angles describing conformation of 2-metylenecyclopentanone unit of enone (σ, ω, τ, a, b, c, d, e, trans1, cis2 and trans2) only one may be appropriate for correlation with the sign of the nπ* transition. This is the torsion angle “b” which assigns the conformer to either the P or M group. Positive sign of “b” predicts the positive nπ* CE and rotatory strength and negative sign of “b” predicts the negative nπ* CE and rotatory strength. Thus, the helicity of 2-methylenecyclopentanone fragment of enone conformer under consideration predicts a sign of the calculated rotatory strength for nπ* transition. In other words, P-helical conformation of the 2-methylenecyclopentanone unit gives rise to a positive nπ* CE and rotatory strength whereas M-helical conformation results in a negative nπ* CE and rotatory strength.
The presented results clearly point to the overriding effect of cyclopentanone conformation over the effects originating from a nonplanarity of enone and ene units.
Our considerations led us to conclude that the rule can be applied for all cis-enones containing 2-methylenecyclopentanone unit. As the conformational analysis shows, the proposed rule works for enones that are practically planar, such as derivatives of bicyclo[2.2.1]heptan-2-one 19–23 (ref. 27) (Chart 3 and Table 6). However, it seems that the rule is not applicable to cis-enones with a π-electron system in which γ carbon atom participates or those with extended conjugation of a double bond.
| Conf. – conformer cmcp – conformation of 2-methylenecyclopentanone unit | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conf. | Torsion angles [°] | nπ* CE | nπ* Rvel | cmcp | |||||||
| σ | ω | τ | a | b | c | d | e | ||||
| a Only one conformer was found.b In methylcyclohexane. | |||||||||||
| P Helical conformers | |||||||||||
| 16(1) | +83.1 | −37.6 | +16.1 | −24.7 | +5.6 | +15.3 | −30.3 | +33.5 | +2.0 | E(5α) | |
| 7c(1) | +100.4 | −21.3 | +0.6 | −11.6 | +29.9 | −36.7 | +30.5 | −12.1 | +6.1 | HC(3β,4α) | |
| 19a | +111.2 | −3.0 | +0.2 | −0.8 | +35.1 | −54.2 | +53.6 | −33.8 | +1.05b | E(4α) | |
| 21a | +108.2 | −1.1 | +0.4 | −0.1 | +35.0 | −53.9 | +52.9 | −34.3 | +0.81b | E(4α) | |
| 20a | +108.2 | −1.1 | +0.2 | 0.0 | +34.9 | −54.0 | +53.8 | −35.1 | +1.14b | E(4α) | |
| 22a | +106.0 | +0.3 | −1.7 | +0.3 | +34.7 | −54.0 | +53.0 | −34.8 | +0.50b | E(4α) | |
| 1(1) | +83.3 | +2.9 | +1.1 | +5.8 | +19.5 | −37.5 | +40.8 | −28.4 | +8.5 | E(4α) | |
| 17(1) | −75.7 | +32.2 | −10.3 | +16.3 | +7.9 | −28.3 | +38.6 | −34.2 | +1.7 | E(5β) | |
| M Helical conformers | |||||||||||
| 5(1) | −104.5 | +24.4 | −5.5 | +15.7 | −27.6 | +29.0 | −20.6 | +3.4 | −6.8 | E(3α) | |
| 4b(1) | −101.0 | +18.5 | −6.4 | +10.0 | −26.6 | +33.0 | −27.9 | +11.4 | −6.2 | HC(3α,4β) | |
| 18(2) | −91.9 | +5.8 | +9.6 | −2.8 | −19.8 | +33.7 | −35.4 | +24.4 | −10.3 | E(4β) | |
| 2(1) | −87.6 | +4.1 | +1.8 | +6.1 | −28.1 | +40.2 | −36.8 | +19.1 | −14.3 | E(4β) | |
| 23a | −109.3 | +0.9 | −0.4 | −0.2 | −34.1 | +53.2 | −53.8 | +35.2 | −1.04b | E(4β) | |
4. Conclusions
In the present report we have examined both experimentally and theoretically the relationship between the stereochemistry and ECD for a wide range of cis-enones with cyclopentanone ring. The main objective of this work was to find a correlation between the sign of the nπ* Cotton effect and the stereochemistry of chromophore or its nearest surroundings. We have found that for enones 1–8 the sign of the nπ* CE can be predicted by some of the existing rules, but none of these rules is applicable in all cases. Hence, it was necessary to find a new, more general rule. As a result of further research undertaken for this purpose, carried out for additional model compounds 13–23, we found a general method for correlation between the structure and the sign of the nπ* CE for cis-enones with 2-methylenecyclopentanone unit. This new rule correlates directly the sign of the torsion angle “b” of the cyclopentanone ring of cis-enones with the sign of the nπ* CE. This means that the positive sign of torsion angle “b” of cyclopentanone ring predicts the positive nπ* CE and vice versa.Our results demonstrated a significant impact of certain substituents at α′ or γ allylic position on nπ* or ππ* rotatory strength values, respectively. As presented, the acetoxyl substituent at α′ position to the ketone carbonyl of enone 5 strongly influences the nπ* transition. However, in enone 8 a strong influence of the conformation of hydroxyl substituent at γ allylic position on the ππ* transition is evident. In this case, a dualistic behavior of hydroxyl substituent, resulting in a positive or negative contribution to the ππ* transition, can be connected with “Z” arrangement of lone pair-O–Cγ transoid–Cβ fragment. As far as we know, this is the first observation of such behavior of substituent in ECD spectra.
Moreover, a detailed analysis of the results showed that (i) the magnitude of the enone torsion angle ω does not correlate with the magnitude of the nπ* rotational strength and the magnitude of ππ* rotational strength; (ii) the sign of the enone torsion angle ω does not correlate with the sign of the ππ* rotational strength; (iii) the sign and the magnitude of ene torsion angle τ does not correlate with neither the sign, nor the magnitude of the nπ* rotational strength, nor the ππ* rotational strength.
Summarizing, the most important finding of this work is that, based solely on the conformational analysis and the consequent sign of the torsion angle “b”, it is possible to determine the conformational chirality of the 2-methylenecyclopentanone ring and thus predict the sign of the nπ* CE. The proposed rule has a more general character because it works also for derivatives of bicyclo[2.2.1]heptan-2-one if they will be treated as having 2-methylenecyclopentanone unit.
Acknowledgements
The authors are indebted to Professor Jacek K. Gawroński (Departament of Chemistry, Adam Mickiewicz University, Poznań, Poland) for providing sample of compound 2 and to Dr Andrzej Zarecki (Institute of Organic Chemistry, Polish Academy of Sciences, Warsaw, Poland) for providing sample of compound 5. This work was supported by the National Science Centre, grant no. UMO-2011/01/B/ST5/06413. All calculations were performed at the Interdisciplinary Centre for Mathematical and Computational Modeling, University of Warsaw (ICM UW), Poland, Grants no. G 36-12 and no. G 34-15.References
- S. F. Brady, M. P. Singh, J. E. Janso and J. Clardy, J. Am. Chem. Soc., 2000, 122, 2116–2117 CrossRef CAS.
- F. Grundschober, Perfum. Flavor., 1979, 4, 15–17 CAS.
- M. Mikołajczyk and P. Bałczewski, Tetrahedron, 1989, 45, 7023–7030 CrossRef.
- M. P. Singh, J. E. Janso, S. W. Luckman, S. F. Brady, J. Clardy, M. Greenstein and W. M. Maiese, J. Antibiot., 2000, 53, 256–261 CrossRef CAS.
- M. Kettering, C. Valdivia, O. Sterner, H. Anke and E. Thines, J. Antibiot., 2005, 58, 390–396 CrossRef CAS PubMed.
- C. Valdivia, M. Kettering, H. Anke, E. Thines and O. Sterner, Tetrahedron, 2005, 61, 9527–9531 CrossRef CAS PubMed.
- D. N. Kirk, Tetrahedron, 1986, 42, 777–818 CrossRef CAS.
- C. Djerassi, R. Records, E. Bunnenberg, K. Mislow and A. Moscowitz, J. Am. Chem. Soc., 1962, 84, 870–872 CrossRef CAS.
- G. Snatzke, Tetrahedron, 1965, 21, 413–419 CrossRef CAS.
- G. Snatzke, Tetrahedron, 1965, 21, 421–438 CrossRef CAS.
- G. Snatzke, Tetrahedron, 1965, 21, 439–448 CrossRef CAS.
- W. B. Whalley, Chem. Ind., 1962, 1024–1027 CAS.
- G. Snatzke, in Optical Rotatory Dispersion and Circular Dichroism in Organic Chemistry, ed. G. Snatzke, Sadtler Research Labs Inc., Philadelphia, 1967, pp. 208–223 Search PubMed.
- G. Snatzke, Angew. Chem., Int. Ed. Engl., 1979, 18, 363–377 CrossRef.
- J. K. Gawroński, Tetrahedron, 1982, 38, 3–26 CrossRef.
- J. Frelek, W. J. Szczepek and H. P. Weiss, Tetrahedron: Asymmetry, 1993, 4, 411–424 CrossRef CAS.
- N. Purdie and H. G. Brittain, Analytical application of circular dichroism, in Techniques and Instrumentation in Analytical Chemistry, Elsevier, Amsterdam – London – New York – Tokyo, 1994, vol. 14 Search PubMed.
- J. K. Gawroński, Conformations, Chiroptical and Related Spectral Properties of Enones, in The Chemistry of Enones, ed. S. Patai and Z. Rappoport, John Wiley & Sons Ltd, New York, 1989, pp. 55–105 Search PubMed.
- D. A. Lightner and J. E. Gurst, Organic Conformational Analysis and Stereochemistry from Circular Dichroism Spectroscopy, Wiley-VCH, New York, 2000 Search PubMed.
- N. Berova, K. Nakanishi and R. W. Woody, Circular Dichroism: Principles and Applications, Wiley-VCH, New York, 2nd edn, 2000 Search PubMed.
- E. Orentas, G. Bagdziunas, U. Berg, A. Zilinskas and E. Butkus, Eur. J. Org. Chem., 2007, 4251–4256 CrossRef CAS.
- M. Kwit, P. Skowronek, J. Gawroński, J. Frelek, M. Woźnica and A. Butkiewicz, Some inherently chiral chromophores – empirical rules and quantum chemical calculations, in Comprehensive Chiroptical Spectroscopy, ed. N. Berova, P. L. Polavarapu, K. Nakanishi and R. W. Woody, John Wiley and Sons, Inc, Hoboken, New Jersey, 2012, vol. 2, pp. 39–72 Search PubMed.
- G. Snatzke, in Fundamental aspects and recent developments in optical rotatory dispersion and circular dichroism, ed. F. Ciardelli and P. Salvadori, Heyden, London, 1973, pp. 109–124 Search PubMed.
- J. Frelek, W. J. Szczepek and H. P. Weiss, Tetrahedron: Asymmetry, 1995, 6, 1419–1430 CrossRef CAS.
- G. Ruecker, K. H. Kahrs and H.-W. Hembeck, Arch. Pharm., 1975, 308, 858–862 CrossRef CAS , see also citation ref. 26.
- J. Frelek, W. J. Szczepek, H. P. Weiss, G. J. Reiss, W. Frank, J. Brechtel, B. Schulthesis and H.-G. Kuball, J. Am. Chem. Soc., 1998, 120, 7010–7019 CrossRef CAS.
- D. A. Lightner, M. J. Flores, B. Vincent Crist and J. K. Gawroński, J. Org. Chem., 1980, 45, 3518–3522 CrossRef CAS.
- E. C. Taylor and Y. Shvo, J. Org. Chem., 1968, 33, 1719–1727 CrossRef CAS.
- S. J. DeSolms, J. Org. Chem., 1976, 41, 2650–2651 CrossRef CAS.
- T. Bakos and I. Vincze, Synth. Commun., 1992, 22, 1377–1383 CrossRef CAS.
- D. N. Kirk, V. Petrow, M. Stansfield and D. M. Williamson, J. Chem. Soc., 1960, 2385–2388 RSC.
- J. Gawroński and A. Gałat, Rocz. Chem., 1975, 49, 91–97 Search PubMed.
- C. W. Shoppee and G. H. R. Summers, J. Chem. Soc., 1952, 2528–2530 RSC.
- R. M. Moriarty and E. S. Wallis, J. Org. Chem., 1959, 24, 1274–1278 CrossRef CAS.
- L. F. Fieser and G. Ourisson, J. Am. Chem. Soc., 1953, 75, 4404–4414 CrossRef CAS.
- M. Anastasia, A. Fiecchi and A. Scala, J. Chem. Soc., Perkin Trans. 1, 1979, 1821–1824 RSC.
- L. F. Fieser and J. E. Herz, J. Am. Chem. Soc., 1953, 75, 121–124 CrossRef CAS.
- O. Wintersteiner and M. Moore, J. Am. Chem. Soc., 1943, 65, 1507–1512 CrossRef CAS.
- O. Wintersteiner and M. Moore, J. Am. Chem. Soc., 1943, 65, 1513–1516 CrossRef CAS.
- M. Kwit, J. Gawroński, D. R. Boyd, N. D. Sharma and M. Kaik, Org. Biomol. Chem., 2010, 8, 5635–5645 CAS.
- HyperChem(TM) Professional 8.0, Hypercube, Inc., Gainesville, FL Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford CT, 2010 Search PubMed.
- G. Scalmani and M. J. Frisch, J. Chem. Phys., 2010, 132, 114110 CrossRef PubMed.
- J. K. Gawroński, Konformacja i czynność optyczna cyklicznych α,β-nienasyconych ketonów, Wydawnictwo Naukowe UAM, Seria Chemia No. 21, Poznań, 1976, p. 94 Search PubMed.
- A. W. Burgstahler and R. C. Barkhurst, J. Am. Chem. Soc., 1970, 92, 7601–7603 CrossRef CAS.
- A. F. Beecham, Tetrahedron, 1971, 27, 5207–5216 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR as well as IR spectra of all synthesized compounds, information about details of calculated UV and CD spectra, total energies, relative energies, and Cartesian coordinates for all optimized structures used in this work. CCDC 882503. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra04991h |
| This journal is © The Royal Society of Chemistry 2014 |