An electrochemical glucose biosensor based on graphene composites: use of dopamine as reducing monomer and as site for covalent immobilization of enzyme†
Huiping Liuab,
Cheng-an Taoa,
Zhihong Hua,
Sida Zhanga,
Jianfang Wang*a and
Yonggong Zhan*b
aCollege of Science, National University of Defense Technology, Changsha 410073, P. R. China. E-mail: wangjianfang@nudt.edu.cn; Fax: +86 731 8457 4250; Tel: +86 731 8457 4241
bCollege of Chemistry and Chemical Engineering, Hunan University, Changsha, 410081, P. R. China. E-mail: ygzhan2006@hnu.edu.cn; Tel: +86 731 88821449
First published on 8th September 2014
Abstract
A new graphene-based electrochemical biosensor was constructed and applied for the simple, rapid and highly selective determination of glucose. The modified glassy carbon electrode (GCE) was prepared using dopamine (DA) as a reducing agent for graphene oxide (GO) and as a capping agent to stabilize and coat the resulting reduced graphene oxide (RGO) surface as it can polymerize to form polydopamine (PDA). PDA-RGO composites were dropped onto a glassy carbon electrode surface, and glucose oxidase (GOx) was immobilized on the surface of the composites by Michael addition. Finally, Pt nanoparticles (PtNPs) were electrodeposited on the modified electrode. Such modified electrodes (denoted as GCE/PDA-RGO/GOx/PtNPs) were characterized by UV-visible spectroscopy, atomic force microscopy, X-ray power diffractometry, Fourier-transform infrared spectroscopy, and scanning electron microscopy. Electrochemical behaviors were investigated by cyclic voltammetry and electrochemical impedance spectroscopy. The fabricated biosensor exhibited a high sensitivity of 33 μA mM−1 cm−2 over a wide linear range of 0.2 mM to 1 mM, good stability, excellent repeatability, and a detection limit of 0.10 mM.
1 Introduction
Fast and efficient methods for the determination of glucose are becoming increasingly important in biology, chemistry, and the food industry; glucose determination is also important in the medical field for controlling diabetes. Diabetes is a worldwide public health problem and one of the leading causes of death and disability in the world.1–4 Numerous studies have been performed to develop simple, sensitive, and accurate approaches for glucose detection, especially those using glucose oxidase (GOx), which has high selectivity and sensitivity.5–7 However, direct electron transfer (DET) without the assistance of mediators for GOx is extremely difficult because of the deep embedding of the redox center of GOx in a protective protein shell.8–10 Several materials have been used to promote electron transfer of redox proteins.11–16 Graphene and its derivatives can facilitate DET of metalloproteins.17–20 Moreover, the excellent conductivity and small band gap of graphene are favorable for transferring electrons from biomolecules.21–23 Reported procedures for fabricating biosensors, which include restoration of the sp2 structure of graphene, preparation of polymer–graphene composites, and immobilization of GOx, are relatively complicated. GOx is generally immobilized by simple physical adsorption, which results in enzyme loss during determination and limits the performance and further application of the enzyme.17,19,24 To develop a more simple and stable fabrication method is very important in the biosensor field.Dopamine (DA), as a biogenic species, has an important physiological function as a chemical messenger in mammals. DA-level abnormalities can result in serious diseases, such as Parkinson's disease.25 Two phenolic hydroxyl groups in DA provide it with reducing power. DA at micromolar-scale concentrations has been used to chemically reduce graphene oxide (GO) into graphene.26,27 Auto-polymerization of DA through air-driven chemical polymerization may also occur to form polydopamine (PDA) at weakly alkaline pH. This characteristic has been used to prepare multifunctional and biocompatible PDA coatings on various surfaces, including graphene.28–34 The functional groups present on the surface of PDA coatings allow covalent immobilization of proteins and enzymes through Michael addition and Schiff base reaction.28 If DA can simultaneously act as both a reductant and monomer to synthesize highly biocompatible PDA-reduced GO composites (PDA-RGO) and if enzymes can be covalently immobilized on the surface of the resultant composites, the preparation of graphene-based biosensors will be conveniently facilitated.
In the present study, DA was used simultaneously as a reducing agent for GO and as a capping agent to stabilize and coat the RGO surface and form biocompatible PDA-RGO composites. A graphene-based electrochemical glucose biosensor was fabricated by modifying the composites on a glassy carbon electrode (GCE) surface. GOx was immobilized on the surface of the composites by Michael addition, and Pt nanoparticles (PtNPs) were electro-deposited on the modified electrode. The fabricated biosensor showed great analytical performance for the detection of glucose, including a high sensitivity of 33 μA mM−1 cm−2 over a wide linear range of 0.2 mM to 1 mM, good stability, excellent repeatability, and a detection limit of 0.10 mM. The proposed fabrication method can promote the preparation of other graphene-based biosensors.
2 Experimental section
2.1 Reagents and apparatus
GOx (50 kU) was purchased from Shanghai Shenggong Reagent Co. Graphite powder and dopamine were purchased from Aladdin. All chemicals were of analytical grade and used as received. All solutions were prepared using ultrapure water.Electrochemical experiments were performed using a CHI660a electrochemical analyzer (Chen Hua Instruments, Shanghai, China). All experiments were carried out at room temperature with a three-electrode system; here, a GCE (Φ = 3 mm), a Ag/AgCl (0.3 M KCl solution) electrode, and a platinum wire served as the working electrode, reference electrode, and auxiliary electrode, respectively. Electrochemical impedance spectroscopy (EIS) was performed in 0.1 M KCl solution containing 5 mM K3[Fe(CN)6]/K4[Fe(CN)6] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at frequencies ranging from 0.01 Hz to 10 kHz at 0.20 V. The amplitude of the applied sine wave potential in each case was 5 mV.
1) at frequencies ranging from 0.01 Hz to 10 kHz at 0.20 V. The amplitude of the applied sine wave potential in each case was 5 mV.
UV-Vis absorption spectra were obtained using a UV-1201 UV-Vis spectrophotometer (China). Atomic force microscopy (AFM) images were acquired in tapping mode using a NanoMan VS instrument (Veeco, USA). X-ray diffractometry (XRD) measurements were performed using a Ttr III type X-ray diffractometer (Rigaku, Japan) equipped with a Cu Kα X-ray radiation (1.54 A) source at θ between 6° and 60°. Scanning electron microscopy (SEM) observations were carried out with a LEO-1503 field emission scanning electron microscope (German). Prior to SEM, the samples were fixed on aluminum stubs and coated with gold powder. X-ray photoelectron spectroscopy (XPS) were carried out with K-Alpha 1063 (Thermo Fisher Scientific, UK); XPSPEAK v4.0.0.0 software (Chemistry, CUHK) was used to perform curve fitting. Fourier transform infrared (FT-IR) spectra were obtained using a Bruker Tensor 27 spectrophotometer within the spectral range of 4000 cm−1 to 400 cm−1 using the KBr pellet method.
2.2 Preparation of PDA-RGO composites
The GO used in this experiment was synthesized from natural graphene powder by a modified Hummers method35,36 and characterized using UV-Vis spectroscopy, optical photography, AFM, XRD and FT-IR spectroscopy. Briefly, 10 mg of GO was suspended in 25 mL of 0.01 M Tris–HCl buffer (pH 8.0). Subsequently, 5 mg of DA powder was added to the GO dispersion after the solution had been deaerated with high-purity nitrogen gas for 10 min. The suspension was ultrasonicated for 10 min in an ice bath and then reacted for 24 h at 60 °C to obtain a black suspension of PDA-RGO. UV-Vis spectroscopy was used to monitor the reduction of GO. The product was obtained by at least three cycles of centrifugation. Finally, the PDA-RGO composite was suspended in 20 mL of pure water.2.3 Construction of GCE/PDA-RGO/GOx/PtNPs modified electrode
Prior to modification, the GCE was carefully polished with 1.0, 0.3, and 0.05 μm γ-alumina powders in sequence and rinsed thoroughly with pure water between each polishing step. The electrode was then ultrasonicated in pure water, ethanol, and pure water for 5 min and dried with nitrogen gas. Six microliters of PDA-RGO suspension was cast onto the surface of the GCE and air-dried. The modified electrode was immersed in GOx in phosphate buffered saline (PBS) solution (10 mg mL−1, pH 8.0) and kept at 4 °C for 4 h to covalently link the GOx to the electrode surface. After washing with pure water, the electrode was immersed into 0.5 M H2SO4 solution containing 2 mM H2PtCl6. Electro-deposition of Pt was carried out by potential scanning from −0.3 V to 0.4 V for 30 cycles.37 The electrode was then washed with pure water and stored at 4 °C prior to use. For comparison, GCE/PDA, GCE/GOx, GCE/PDA-RGO, GCE/PDA-RGO/GOx, and GCE/PDA-RGO/PtNP electrodes (“/” represents which are used to modify electrodes in successive steps, “-” means which are mixed first, and then use to modify electrodes) were prepared using a similar method.3 Results and discussion
3.1 Characterization of the nanocomposites
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and the carboxylate carbon (C(O)O). After reduced and composite with PDA, although the C1s XPS spectrum of PDA-RGO composite still exhibits these same oxygen-containing groups, their peak intensities are much smaller than those in GO. Moreover, there is an additional component at 285.7 eV corresponding to C–N bond.38 These observation indicates considerable de-oxygenation by DA reduction, which is consistent with the UV-Vis results.
O), and the carboxylate carbon (C(O)O). After reduced and composite with PDA, although the C1s XPS spectrum of PDA-RGO composite still exhibits these same oxygen-containing groups, their peak intensities are much smaller than those in GO. Moreover, there is an additional component at 285.7 eV corresponding to C–N bond.38 These observation indicates considerable de-oxygenation by DA reduction, which is consistent with the UV-Vis results.
AFM, XRD and FT-IR were used to further confirm the formation of PDA-RGO composites. Fig. 2 shows a typical AFM image obtained in tapping mode displaying the morphology and thickness of GO and PDA-RGO. Compared with GO, the large RGO sheet of the composite was coated with numerous small and uniformly distributed island-like nanostructures, which are PDA coatings. The corresponding line-scan indicated that the thickness of PDA-RGO is 1.76 nm, much larger than that of GO (∼1 nm). These results could be attributed to the presence of a PDA coating on both sides of the RGO sheets.
 | ||
| Fig. 2 Typical AFM images of (A) GO and (B) PDA-RGO. Cross-sectional analysis was performed along the lines shown in the AFM images. | ||
The XRD patterns of PDA-RGO composite are shown in Fig. 3A. For comparison, the XRD patterns of DA, PDA and GO are also displayed. DA powder showed many peaks. While once it has polymerized to be PDA, only a wide diffraction peak exist around 24° due to the stacking of the benzene rings of DA molecules. GO has the characteristic peak at 10.0°, while the PDA-RGO composite only showed a wide peak at 24°, which is very similar to that of pure PDA and demonstrates the DA has successfully self-polymerized. The disappearance of the peak at 10.0° also suggests that GO was thoroughly converted to RGO.
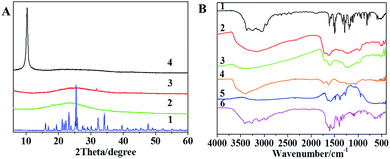 | ||
| Fig. 3 (A) XRD patterns of (1) DA, (2) PDA, (3) PDA-RGO, and (4) GO. (B) FT-IR spectra of (1) DA, (2) PDA, (3) PDA-RGO, (4) GO, (5) PDA-RGO/GOx, and (6) GOx. | ||
The FT-IR spectra of DA, PDA and PDA-RGO are displayed in Fig. 3B. DA shows broad and strong bands in the 3000–3400 cm−1 region (aromatic O–H stretching vibration). Peaks at 1519 cm−1 (NH2 scissoring vibration), 1627 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration), 1340 cm−1 (CH2 bending vibration), 1175 cm−1 (C–C stretching vibration), 1260 cm−1 (C–O–H symmetry vibration), and 1190 cm−1 (C–O symmetry vibration) were also observed.37 After polymerization, one broad band was observed at the 3000–3400 cm−1 region and the peaks at 1519, 1340, 1175, 1260, and 1190 cm−1 disappeared. A new peak at 1707 cm−1 (C
C stretching vibration), 1340 cm−1 (CH2 bending vibration), 1175 cm−1 (C–C stretching vibration), 1260 cm−1 (C–O–H symmetry vibration), and 1190 cm−1 (C–O symmetry vibration) were also observed.37 After polymerization, one broad band was observed at the 3000–3400 cm−1 region and the peaks at 1519, 1340, 1175, 1260, and 1190 cm−1 disappeared. A new peak at 1707 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) appeared. These results demonstrate that DA underwent intramolecular cyclization to form indole derivatives (Fig. S1†). The IR spectra of PDA-RGO are similar to those of DA; the absence of peaks at 1731 cm−1 (C
O) appeared. These results demonstrate that DA underwent intramolecular cyclization to form indole derivatives (Fig. S1†). The IR spectra of PDA-RGO are similar to those of DA; the absence of peaks at 1731 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1226 cm−1 (C–O–C), and 1054 cm−1 (C–O) was observed, which further implies that reduction and polymerization simultaneously occurred to successfully yield the PDA-RGO composites.
O), 1226 cm−1 (C–O–C), and 1054 cm−1 (C–O) was observed, which further implies that reduction and polymerization simultaneously occurred to successfully yield the PDA-RGO composites.
The surface morphology of the PDA-RGO composite was examined by SEM, as illustrated in Fig. 4. PDA-RGO (Fig. 4B) has a typical crumpled and wrinkled sheet structure, very similar to that of GO (Fig. 4C). The SEM image of PDA (Fig. 4A) shows a very rough surface featuring several irregular cracks.39 This result indicates that the PDA layer coating RGO is very thin and therefore cannot affect the morphology of RGO significantly.
 | ||
| Fig. 4 SEM images of (A) PDA, (B) PDA-RGO, (C) GO, (D) PDA-RGO/GOx on a Si substrate, and (E) PDA-RGO/GOx/PtNPs on the GCE electrode. (F) EDX spectra of PDA-RGO/GOx/PtNPs (1 bar = 10 μm). | ||
Finally, EIS was used to study the interface properties of electrodes modified with the PDA-RGO composites (Fig. 5).40 Compared with GCE, when GO is coated on the electrode, a rapid increase in the diameter of the semicircle is obtained because of the ability of GO to block electron transfer. When the PDA-RGO composite was deposited on the GCE, the Rct dramatically decreased to 3000 Ω, which suggests that PDA-RGO can greatly improve the conductivity of the resultant product and promote electron transfer. These observations are attributed to the unique properties of RGO.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of PDA-RGO disappeared, which implies successful immobilization of GOx through Michael addition (Fig. S2†). The SEM image of PDA-RGO/GOx (Fig. 4D) shows no obvious difference from that of PDA-RGO (Fig. 4B), likely because the GOx particles are too small in size to be observed under the present conditions. To confirm the immobilization of GOx, we studied the EIS spectra of GCE/GOx and GCE/PDA-RGO/GOx electrodes. As shown in Fig. 5, the Rct of GOx (1000 Ω) is larger than that of the bare GCE, which demonstrates inhibition of electron transfer by GOx. When GOx is covalently linked to the surface of GCE/PDA-RGO, the Rct increases from 3000 Ω to 5000 Ω. It illustrates that GOx is steadily immobilized on the modified electrode.
O) of PDA-RGO disappeared, which implies successful immobilization of GOx through Michael addition (Fig. S2†). The SEM image of PDA-RGO/GOx (Fig. 4D) shows no obvious difference from that of PDA-RGO (Fig. 4B), likely because the GOx particles are too small in size to be observed under the present conditions. To confirm the immobilization of GOx, we studied the EIS spectra of GCE/GOx and GCE/PDA-RGO/GOx electrodes. As shown in Fig. 5, the Rct of GOx (1000 Ω) is larger than that of the bare GCE, which demonstrates inhibition of electron transfer by GOx. When GOx is covalently linked to the surface of GCE/PDA-RGO, the Rct increases from 3000 Ω to 5000 Ω. It illustrates that GOx is steadily immobilized on the modified electrode.3.2 Direct electrochemistry of GOx immobilized on the GCE/PDA-RGO/GOx/PtNP electrode
DET of GOx-modified electrodes was studied by cyclic voltammetry (CV) in PBS solution without O2 (Fig. 6). No current peak was observed in the voltammograms of GCE/PDA, GCE/PDA-RGO and GCE/PDA-RGO/PtNPs. The absence of a peak indicates that PDA, PDA-RGO and PDA-RGO/PtNPs composites are inactive electrochemically. The background current of the RGO-modified electrodes obviously increased, which can be ascribed to the large specific surface area and extraordinary electron transfer conductivity of the material. No redox peak current attributable to GOx was observed on the GCE/GOx electrode, which suggests that achieving DET in the electrodes is extremely difficult because of the deep embedding of the redox center of GOx in a protective protein shell.8–10 The CV curves of GOx on the GCE/PDA-RGO/GOx electrode displays a pair of stable redox peaks, which is attributable to the redox reaction of the electroactive center of GOx. This finding indicates that the large surface-to-volume ratio and high conductivity of RGO works together with the good biocompatibility of PDA to promote DET between GOx and the surface of the electrodes.41 Two well-defined redox peaks were obtained for the GCE/PDA-RGO/GOx/PtNPs electrode with Epa = −0.546 V and Epc = −0.617 V. The peak-to-peak separation (ΔEp) was approximately 71 mV, much smaller than that observed for the GCE/PDA-RGO/GOx electrode (104 mV). This result reveals that a larger number of reversible electron transfers in the redox active center of the GOx were achieved, which may be attributed to the excellent conductivity of PtNPs and the synergistic effects of PtNPs and RGO. In the presence of O2, glucose standard was added to the electrodes, among which the GCE/PDA-RGO/GOD/PtNP electrode showed the best responses (Fig. S4†). | ||
| Fig. 6 CV curves of the GCEs modified with (a) GOx, (b) PDA, (c) PDA-RGO, (d) PDA-RGO/PtNPs, (e) PDA-RGO/GOx, and (f) PDA-RGO/GOx/PtNP films in PBS saturated with N2 at a scan rate of 0.1 V s−1. | ||
The influence of scan rate on the peak current was also investigated, as shown in Fig. 7. As the scan rate increased from 20 mV s−1 to 300 mV s−1, the redox peak currents linearly increased, which implies that the electrochemical kinetics is a surface-controlled process. According to Faraday's law, the average surface concentration of electroactive GOx (Γ*) immobilized on the GCE/PDA-RGO/GOx/PtNPs electrode may be estimated to be 3.1 × 10−9 mol cm−2, which is a value characteristic of a small molecule (FAD) in contrast to the enzyme which should be around 10−11 mol cm−2.10,42 It demonstrates that free FAD is extruded from the enzyme and remains adsorbed on the electrode surface and GOx is immobilized on the surface of PDA-RGO through covalent linkage. These results are in agreement with the FT-IR analysis.
3.3 Glucose detection using the GCE/PDA-RGO/GOx/PtNP electrode
Fig. 8A shows CV curves of the GCE/PDA-RGO/GOx/PtNP electrode in 0.01 M PBS solution containing different concentrations of glucose and saturated with air. Successive addition of glucose resulted in a gradual decrease in reduction current. This trend could be explained by the fact that addition of glucose triggers the enzyme-catalyzed reaction of GOx and glucose. This reaction causes a decrease in the amount of oxidized GOx on the GCE/PDA-RGO/GOx/PtNPs electrode and reduces the electrode reduction current (see ESI†). The response current was directly proportional to the concentration of glucose in the range of 0.2–1 mM, with a correlation coefficient (R2) of 0.9957 (Fig. 8B). The relative standard deviation of each point is less than 0.65%. The biosensor also displayed a high sensitivity of approximately 33 μA mM−1 cm−2.The detection limit of the biosensor was 0.10 mM based on a signal-to-noise ratio of 3 (inset in Fig. 8A). Results indicate that the modified electrode has satisfactory performance and may be practically applied in the determination of human blood sugar concentrations.
3.4 Reproducibility, selectivity, and long-term stability of the biosensor
The prepared biosensor displayed good reproducibility. The RSD of the current response to 0.2 mM glucose was 2.7% in five successive measurements (Fig. S5†). After 50 cycles of potential scanning between −0.8 and 0 V, the cathodic current remained almost unchanged, with an RSD of 0.8% (Fig. S6a and b†). The RSDs of the peak current response of four independent electrodes to 0.2 mM glucose was 3.3% (Fig. S6†). The effect of interferences on the glucose response was further studied to evaluate the selectivity of the sensor (Fig. S7†). Ascorbic acid (AA) and DA are common reducing substances in blood and they were chosen as the interference molecules. It's found that both of AA and DA has little effect on the glucose response. When there are only 0.2 mM glucose in the PBS solution, the current response reached to 40.39 μA. When ten-fold concentration of AA (2 mM) co-exists in the solution, the current response only changed a little (retained 98%). DA got the similar results. These results indicate that the proposed biosensor has high selectivity.The stability of the biosensor was finally investigated. The response current maintained 91% of its initial electrochemical response after storage at 4 °C for one week (Fig. S6c†). This stability is attributed to the unique properties of RGO, efficient covalent linkages between GOx and PDA, and the synergistic effects of PtNPs and RGO. These results further indicate that the PDA-RGO composite could efficiently immobilize GOx, retain its bioactivity, and prevent biomolecules from leaking out. These actions are attributable to chemical interactions between GOx and the electrode, rather than pure physical adsorption.
4 Conclusions
An electrochemical glucose biosensor based on polymer–graphene/enzyme/Pt composites was fabricated. Here, DA served as both a reducing monomer and site for covalent immobilization of enzyme. DA simultaneously reduced GO and capped the resultant RGO to form PDA-RGO composites, which could easily and covalently immobilize enzymes through Michael addition. The biosensor exhibited good sensitivity, excellent stability, and superior repeatability toward glucose. These characteristics are attributed to the large surface area of the electrode, the fast electron transfer of RGO, the synergistic effects of PtNPs and RGO, and the stable immobilization of GOx. The proposed procedure for fabricating graphene-based biosensors is convenient and the performance of the resultant sensor is excellent. These findings indicate that the proposed biosensor has potential applications in glucose sensing.Acknowledgements
This work was supported by the National Natural Science Foundation of China (61171020, 21203247, 61376125).Notes and references
- A. L. Ghindilis, P. Atanasov and E. Wilkins, Electroanalysis, 1997, 9, 661 CrossRef CAS PubMed.
- P. Pandey, S. P. Singh and S. K. Arya, Langmuir, 2007, 23, 3333 CrossRef CAS PubMed.
- R. W. Keay and C. J. McNeil, Biosens. Bioelectron., 1998, 13, 963 CrossRef CAS.
- J. J. Xu and H. Y. Chen, Anal. Chim. Acta, 2000, 423, 101 CrossRef CAS.
- N. S. Oliver, C. Toumazou, A. E. Cass and D. G. Johnston, Diabetic Med., 2009, 26, 197 CrossRef CAS PubMed.
- J. Wang, Chem. Rev., 2008, 108, 814 CrossRef CAS PubMed.
- C. Chen, Q. Xie and D. Yang, RSC Adv., 2013, 3, 4473 RSC.
- A. Guiseppi-Elie, C. Lei and R. H. Baughman, Nanotechnology, 2002, 13, 559 CrossRef CAS.
- O. Nadzhafova, M. Etienne and A. Walcarius, Electrochem. Commun., 2007, 9, 1189 CrossRef CAS PubMed.
- J. M. Goran, S. M. Mantilla and K. J. Stevenson, Anal. Chem., 2013, 85, 1571 CrossRef CAS PubMed.
- C. Lei and J. Deng, Anal. Chem., 1996, 68, 3344 CrossRef CAS.
- S. Kroning, F. W. Scheller and U. Wollenberger, Electroanalysis, 2004, 16, 253 CrossRef PubMed.
- S. Gaspar, H. Zimmermann and I. Gazaryan, Electroanalysis, 2001, 12, 284 CrossRef.
- Z. H. Dai, S. Q. Liu and H. X. Ju, Biosens. Bioelectron., 2004, 19, 861 CrossRef CAS PubMed.
- J. S. Long, D. S. Silvester and G. G. Wildgoose, Bioelectrochemistry, 2008, 74, 183 CrossRef CAS PubMed.
- X. B. Lu, Q. Zhang and L. Zhang, Electrochem. Commun., 2006, 8, 874 CrossRef CAS PubMed.
- X. Kang, J. Wang and H. Wu, Biosens. Bioelectron., 2009, 25, 901 CrossRef CAS PubMed.
- P. Wu, Q. Shao, Y. Hu and J. Jin, Electrochim. Acta, 2010, 55, 8606 CrossRef CAS PubMed.
- C. Ruan, T. Li and Q. Niu, Electrochim. Acta, 2012, 64, 183 CrossRef CAS PubMed.
- H. Xu, H. Dai and G. Chen, Talanta, 2010, 81, 334 CrossRef CAS PubMed.
- Y. Liu, X. Dong and P. Chen, Chem. Soc. Rev., 2012, 41, 2283 RSC.
- D. Chen, L. Tang and J. Li, Chem. Soc. Rev., 2010, 39, 3157 RSC.
- T. Gan and S. Hu, Microchim. Acta, 2011, 175, 1 CrossRef CAS.
- K. Mao, D. Wu and Y. Li, Anal. Biochem., 2012, 422, 22 CrossRef CAS PubMed.
- A. Pezzella, M. d'Ischia and A. Napolitano, J. Med. Chem., 1997, 40, 2211 CrossRef CAS PubMed.
- H. Liu, P. Xi and G. Xie, J. Phys. Chem. C, 2012, 116, 3334 CAS.
- I. Kaminska, M. R. Das and Y. Coffinier, ACS Appl. Mater. Interfaces, 2012, 4, 1016 CAS.
- L. Q. Xu, W. J. Yang and K.-G. Neoh, Macromolecules, 2010, 43, 8336 CrossRef CAS.
- H. Lee, S. M. Dellatore and W. M. Miller, Science, 2007, 318, 426 CrossRef CAS PubMed.
- S. Zurcher, D. Wackerlin and Y. Bethuel, J. Am. Chem. Soc., 2006, 128, 1064 CrossRef PubMed.
- M. A. Watson, J. Lyskawa and C. Zobrist, Langmuir, 2010, 26, 15920 CrossRef CAS PubMed.
- S. Alwarappan, A. Erdem and C. Liu, J. Phys. Chem. C, 2009, 113, 8853 CAS.
- N. G. Shang, P. Papakonstantinou and M. McMullan, Adv. Funct. Mater., 2008, 18, 3506 CrossRef CAS PubMed.
- Y. Wang, Y. Li, L. Tang and J. Lu, Electrochem. Commun., 2009, 11, 889 CrossRef CAS PubMed.
- D. C. Marcano, D. V. Kosynkin and J. M. Berlin, ACS Nano, 2010, 4, 4806 CrossRef CAS PubMed.
- C. Tao, J. Wang and Y. Lv, J. Mater. Chem., 2012, 22, 24856–24861 RSC.
- X. Q. Lin and Y. X. Li, Biosens. Bioelectron., 2006, 22, 253 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhamme, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558 CrossRef CAS PubMed.
- F. Yu, S. Chen and Y. Chen, J. Mol. Struct., 2010, 982, 152 CrossRef CAS PubMed.
- R. Ehret, W. Baumann and M. Brischwein, Biosens. Bioelectron., 1997, 12, 29 CrossRef CAS.
- B. Karlberg, M. Grasserbauer and J. E. T. Andersen, Trends Anal. Chem., 2009, 28, 515 CrossRef CAS PubMed.
- P. Wu, Q. Shao and Y. J. Hu, Electrochim. Acta, 2010, 55, 8606 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The UV-Vis absorption spectra of PDA-RGO. The size of PtNPs. CVs of different modified electrodes in the absence and in the presence of glucose. The characterization of reproducibility, selectivity, and long-term stability of biosensor. See DOI: 10.1039/c4ra04975f |
| This journal is © The Royal Society of Chemistry 2014 |




